- +86-0533-2185556
- WhatsApp: +86 15965530500
- admin@hangyubiotech.com
Your Location:Home >Products >small-molecule inhibitor >112163-33-4
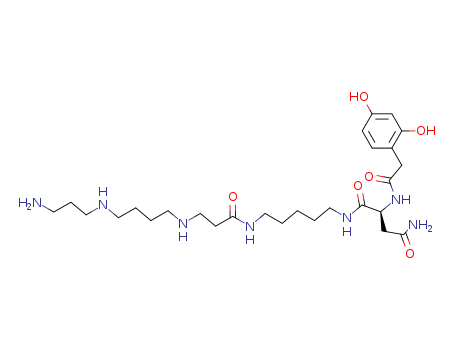

Purity:99%
|
Side effects |
Lactoferrin is safe in amounts consumed in food. Consuming higher amounts of lactoferrin from cow's milk might also be safe for up to a year. Human lactoferrin that is made from specially processed rice appears to be safe for up to 14 days. Lactoferrin can cause diarrhea. In very high doses, skin rash, loss of appetite, fatigue, chills, and constipation have been reported. |
|
description |
Lactoferrin (formerly known as lactotransferrin) is a glycoprotein, and a member of a transferrin family, thus belonging to those proteins capable of binding and transferring Fe3+ ions (Metz-Boutique et al., 1984).It is produced by various mammals and is found in milk, nasal secretions, saliva and tears, and it is in great abundance in human colostrum. It has intrigued scientists for decades.Lactoferrin was first isolated by Sorensen and Sorensen from bovine milk in 1939. In 1960 it was concurrently determined to be the main iron binding protein in human milk by three independent laboratories (Groves, 1960; Johanson, 1960; Montreuil et al., 1960).Subsequent research identified lactoferrin in secretions from exocrine glands and in specific granules of neutrophils. Neutrophils after degranulation were obser ved to be the main source of lactoferrin in blood plasma (Iyer and Lonnerdal, 1993).Due to the increase in its concentration during most inflammatory reactions and some viral in fections, several authors classify lactoferrin as an acute-phase protein (Kanyshkova et al., 2001). Its concentration increases in all biological fluids, but the highest levels have been detected in the nidus of inflammation (Birgens, 1985).Thus, lactoferrin has a wide variety of biological functions, many of which do not appear to be connected with its iron binding ability (Brock, 2002). |
|
Definition |
Lactoferrin is a protein found in cow milk and human milk. Colostrum, the first milk produced after a baby is born, contains high levels of lactoferrin, about seven times the amount found in milk produced later on. Lactoferrin is also found in fluids in the eye, nose, respiratory tract, intestine, and elsewhere. People use lactoferrin as medicine. |
InChI:InChI=1/C27H47N7O6/c28-10-6-13-30-11-4-5-12-31-16-9-25(38)32-14-2-1-3-15-33-27(40)22(19-24(29)37)34-26(39)17-20-7-8-21(35)18-23(20)36/h7-8,18,22,30-31,35-36H,1-6,9-17,19,28H2,(H2,29,37)(H,32,38)(H,33,40)(H,34,39)/t22-/m0/s1
An efficient and versatile synthesis of ...
![[3-(4-{Benzyloxycarbonyl-[2-(5-{(S)-2-[2-(2,4-bis-benzyloxy-phenyl)-acetylamino]-3-carbamoyl-propionylamino}-pentylcarbamoyl)-ethyl]-amino}-butylamino)-propyl]-carbamic acid benzyl ester](/upload/2025/4/2e29621c-dc9b-470c-bb36-bb9354a3128e.png)
[3-(4-{Benzyloxycarbonyl-[2-(5-{(S)-2-[2-(2,4-bis-benzyloxy-phenyl)-acetylamino]-3-carbamoyl-propionylamino}-pentylcarbamoyl)-ethyl]-amino}-butylamino)-propyl]-carbamic acid benzyl ester

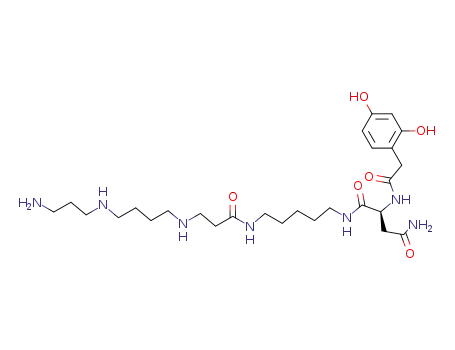
JSTX 3
| Conditions | Yield |
|---|---|
|
With
hydrogen; acetic acid;
palladium dihydroxide;
at 20 ℃;
for 3h;
|
![N-[N<sup>α</sup>-(tert-butoxycarbonyl)-L-asparaginyl]-1,5-diaminopentane](/upload/2025/4/0d6961f4-4650-4ee7-b1d9-a0addf7c75bd.png)
N-[Nα-(tert-butoxycarbonyl)-L-asparaginyl]-1,5-diaminopentane


JSTX 3
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 6 steps
1: dimethylformamide / 0.5 h / 20 °C
2: 92 percent / Cs2CO3 / dimethylformamide / 1 h / 50 °C
3: TFA / CHCl3 / 1 h / 0 - 20 °C
4: Et3N / dimethylformamide / 2 h / 20 °C
5: 2-mercaptoethanol; DBU / dimethylformamide / 0.5 h / 20 °C
6: H2; AcOH / Pd(OH)2 / 3 h / 20 °C
With
hydrogen; caesium carbonate; acetic acid; 1,8-diazabicyclo[5.4.0]undec-7-ene; triethylamine; trifluoroacetic acid; 2-hydroxyethanethiol;
palladium dihydroxide;
In
chloroform; N,N-dimethyl-formamide;
|
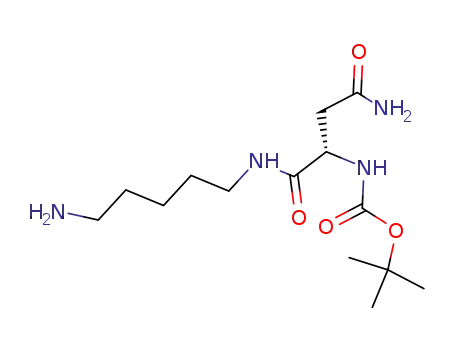
N-[Nα-(tert-butoxycarbonyl)-L-asparaginyl]-1,5-diaminopentane
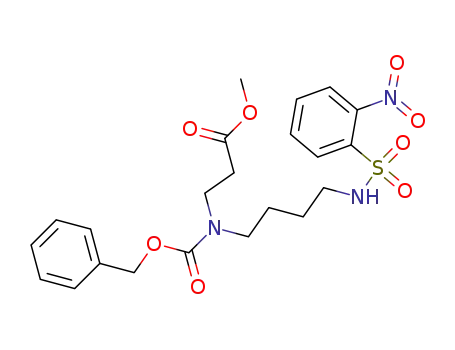
methyl 8-((2-nitrobenzenesulfonyl)amino)-4-(benzyloxycarbonyl)-4-azaoctanoate
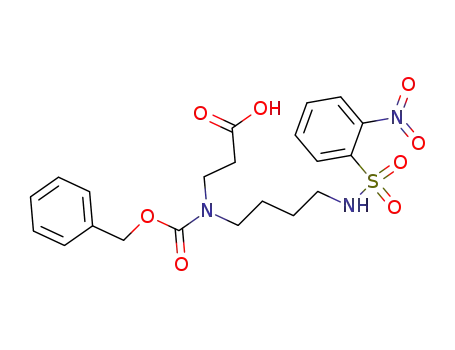
8-((2-nitrobenzenesulfonyl)amino)-4-(benzyloxycarbonyl)-4-azaoctanoic acid
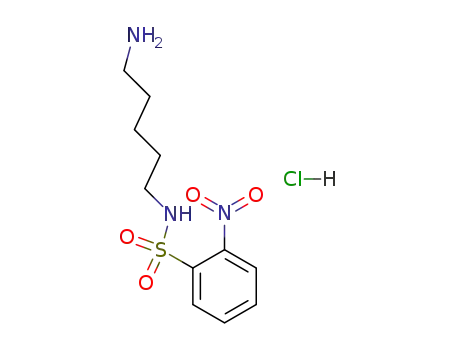
mono-2-nitrobenzenesulfonamide-cadaverine HCl salt
CAS:112-84-5
CAS:114153-91-2
CAS:112-34-5