- +86-0533-2185556
- WhatsApp: +86 15965530500
- admin@hangyubiotech.com
Your Location:Home >Products >Pharmaceutical intermediate >1070-89-9
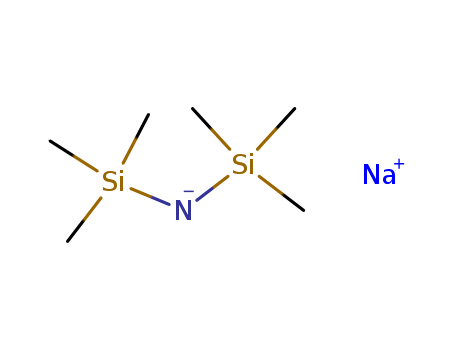

pd_meltingpoint:171-175 °C
Appearance:Slightly yellow to light beige crystalline powder
Purity:99%
|
Physical properties |
mp 171–175 °C; bp 170 °C/2 mmHg. |
|
General Description |
Sodium bis(trimethylsilyl)amide solution (NaHMDS) is widely used as a strong base in organic synthesis for deprotonation reactions and base-catalyzed reactions. It is also involved in the generation of enolates, Wittig reagents and carbenes. |
|
Purification Methods |
It can be sublimed at 170o/2mm (bath temperature 220-250o) onto a cold finger, and can be recrystallised from *C6H6 (its solubility is: 10g in 100mL at 60o). It is slightly soluble in Et2O and is decomposed by H2O. [Wannagat & Niederprüm Chem Ber 94 1540 1961.]It is available commercially under N2 in Sure/Seal bottles in tetrahydrofuran (various concentrations) and at ~0.6M in toluene. [Beilstein 4 IV 4014.] |
InChI:InChI=1/C6H19NSi2.Na/c1-8(2,3)7-9(4,5)6;/h7H,1-6H3;/q;+1
The divalent bis(trimethylsilyl)amide co...
-
After isolating an unusual binuclear, bu...
The 1,8-bis(4′,4′-dimethyloxazolin-2′-yl...
The reaction of sodium bis(trimethylsily...
A series of mono- and bimetallic Ni alky...
The method of continuous variation in co...
[U(ODtbp)3] (ODtbp?=?O-2,6-tBu2C6H3) rea...
It is shown that the deprotonation of bu...
Ketone enolization by sodium hexamethyld...
1,1,1,3,3,3-hexamethyl-disilazane

sodium hexamethyldisilazane
| Conditions | Yield |
|---|---|
|
With
sodium hydride; sodium t-butanolate;
In
toluene;
for 48h;
Reflux;
|
86% |
|
With
sodium amide;
In
toluene;
Inert atmosphere;
|
|
|
With
styrene; sodium;
|
|
|
With
sodium amide;
In
hexane;
Glovebox;
Inert atmosphere;
|
|
|
With
potassium hydride;
In
toluene;
Inert atmosphere;
|
|
|
With
butylsodium;
In
hexane;
at 20 ℃;
for 2.16667h;
Inert atmosphere;
Schlenk technique;
Glovebox;
|
|
|
With
sodium; triethylamine; isoprene;
at 20 ℃;
for 3h;
|
lithium bis(trimethylsilyl)amide diethyl etherate


sodium hexamethyldisilazane
| Conditions | Yield |
|---|---|
|
With
sodium t-butanolate;
In
benzene;
at 20 ℃;
|
77% |
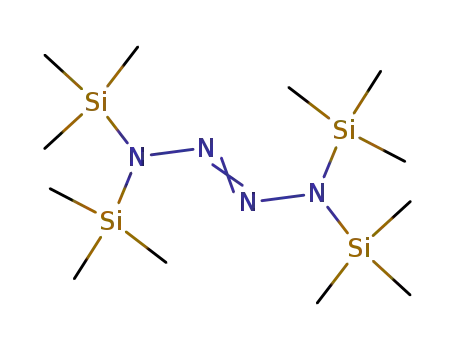
tetrakis(trimethylsilyl)tetrazene
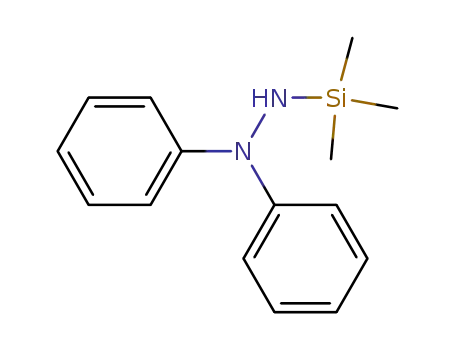
N,N-diphenyl-N'-(trimethylsilyl)hydrazine

1,1,1,3,3,3-hexamethyl-disilazane
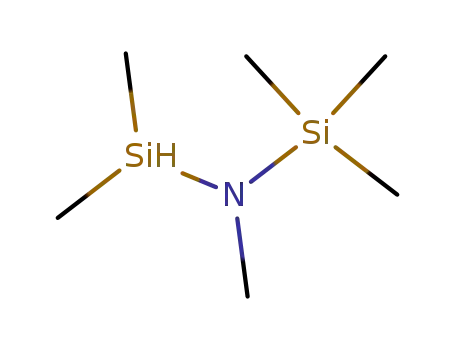
hexamethyldisilazan
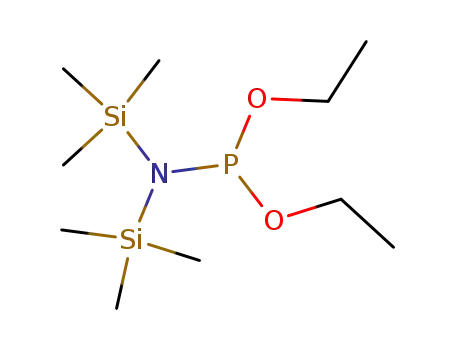
diethoxy
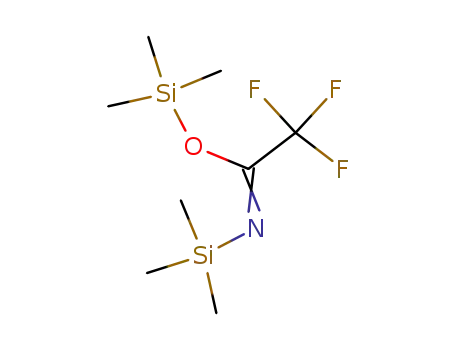
N,O-Bis(trimethylsilyl)trifluoroacetamide
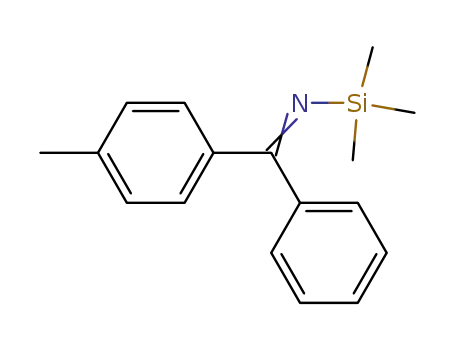
4-Methyl-N-trimethylsilyl-benzophenonimin
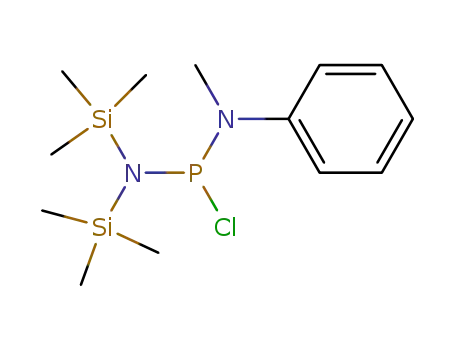
C13H26ClN2PSi2
CAS:15901-40-3
CAS:10196-49-3
CAS:52617-99-9