- +86-0533-2185556
- WhatsApp: +86 15965530500
- admin@hangyubiotech.com
Your Location:Home >Products >API >144481-98-1

pd_meltingpoint:122-127oC
Appearance:White crystalline powder
Purity:99%
|
Synthesis |
The synthesis of landiolol appeared in an earlier patent in 1990. Esterification of 3- (4-hydroxyphenyl)propionic acid (141) with 2,2-dimethyl- 1,3-dioxolan-4-ylmethyl chloride (142) in DMSO gave desired ester 143 in 57% yield. Treatment of phenol 143 with bromo epoxide 144 in the present of K2CO3 afforded ether 145 in 76% yield. Epoxide 145 was then reacted with free amine 146 via a neucleophilic ring opening process to provide landiolol (14). |
InChI:InChI=1/C25H39N3O8.ClH/c1-25(2)35-18-22(36-25)17-34-23(30)8-5-19-3-6-21(7-4-19)33-16-20(29)15-26-9-10-27-24(31)28-11-13-32-14-12-28;/h3-4,6-7,20,22,26,29H,5,8-18H2,1-2H3,(H,27,31);1H/t20-,22+;/m0./s1
The invention discloses a preparation me...
The invention discloses a preparation me...
The invention belongs to the field of ph...
PROBLEM TO BE SOLVED: To provide a proce...
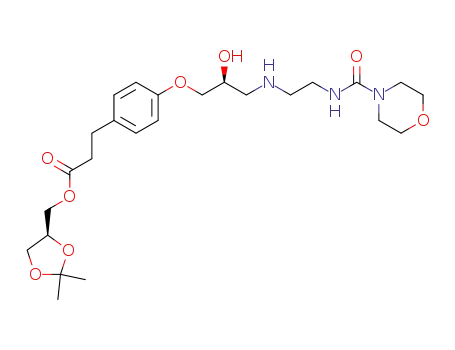
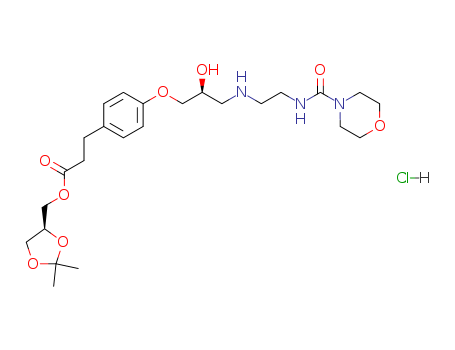
2,2-dimethyl-1,3-dioxolan-4S-ylmethyl 3-<4-<3-<2-(morpholinocarbonylamino)ethylamino>-2S-hydroxypropoxy>phenyl>propionate

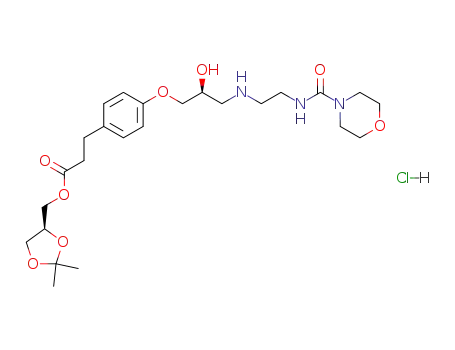
Landiolol hydrochloride
| Conditions | Yield |
|---|---|
|
With hydrogenchloride; In ethyl acetate; at 5 - 10 ℃; for 2h;
|
95.9% |
|
With hydrogenchloride; In isopropyl alcohol;
|
74.7% |
|
With hydrogenchloride; In isopropyl alcohol;
|
74.7% |
![[(4S)-2,2-dimethyl-1,3-dioxolan-4-yl]methyl-3-{4-[(2S)-2-hydroxy-3-[(2-{[(morpholin-4-yl)carbonyl]amino}ethyl)amino]propoxy]phenyl}propanoate oxalate](/upload/2025/4/fe5766b7-9a40-4100-9fee-d574a6ee1184.png)
[(4S)-2,2-dimethyl-1,3-dioxolan-4-yl]methyl-3-{4-[(2S)-2-hydroxy-3-[(2-{[(morpholin-4-yl)carbonyl]amino}ethyl)amino]propoxy]phenyl}propanoate oxalate


Landiolol hydrochloride
| Conditions | Yield |
|---|---|
|
With pyridine hydrochloride; sodium hydroxide; In water; isopropyl alcohol; at 0 - 50 ℃; for 16h; pH=8.5; Solvent; Reagent/catalyst; Temperature;
|
85.2% |
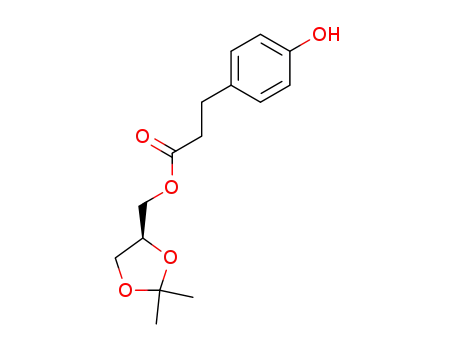
3-(4-hydroxyphenyl)propionic acid [(4S)-2,2-dimethyl-1,3-dioxolan-4-yl]methyl ester
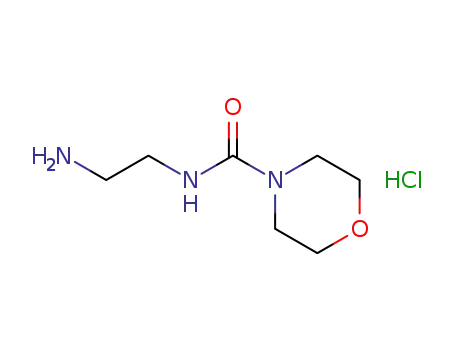
2-(morpholine-4-carboxamido)ethanamino hydrochloride

2,2-dimethyl-1,3-dioxolan-4S-ylmethyl 3-<4-<3-<2-(morpholinocarbonylamino)ethylamino>-2S-hydroxypropoxy>phenyl>propionate
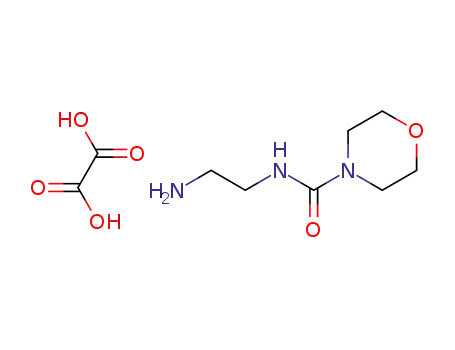
N-(2-aminoethyl)-4-morpholine carboxamide oxalic acid
CAS:118685-33-9
CAS:6138-41-6
CAS:109-78-4
CAS:11006-90-9