- +86-0533-2185556
- WhatsApp: +86 15965530500
- admin@hangyubiotech.com
Your Location:Home >Products >API >1000339-23-0

Purity:99%
|
General Description |
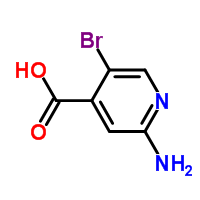
2-Amino-5-bromoisonicotinic acid is a specialized chemical compound that carries the molecular formula C6H5BrN2O2. It is categorized as an isonicotinic acid, which belongs to a class of organic compounds known as pyridinecarboxylic acids. This specific compound is recognized for its uses in various types of chemical and pharmaceutical research due to the unique properties it holds. It is characterized by its bromo and amino substituents which can interact in reactions, giving it utility as a building block or precursor in various types of synthesis. As with many chemicals, it should be handled with care due to potential risks upon contact or ingestion. It is notable for being an advanced intermediate in the synthesis of more complex molecules. |
InChI:InChI=1/C6H5BrN2O2/c7-4-2-9-5(8)1-3(4)6(10)11/h1-2H,(H2,8,9)(H,10,11)
A class of compounds that inhibit Hepati...
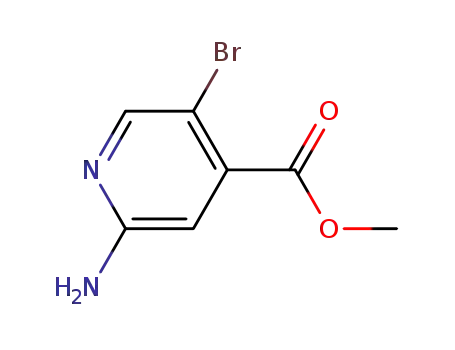
methyl 2-amino-5-bromo-pyridine-4-carboxylate

C6H5BrN2O2
| Conditions | Yield |
|---|---|
|
methyl 2-amino-5-bromopyridine-4-carboxylate; With water; lithium hydroxide; In tetrahydrofuran; methanol; at 70 ℃; for 2h; Inert atmosphere;
With hydrogenchloride; In tetrahydrofuran; methanol; water; at 0 ℃; pH=6;
|
83% |
C6H5BrN2O2

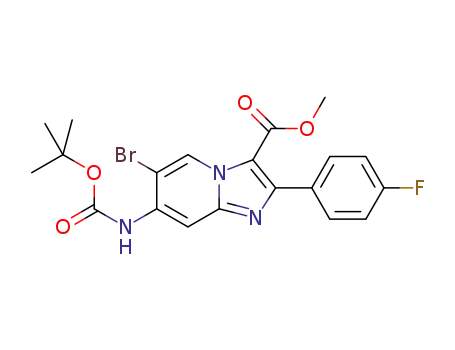
C20H19BrFN3O4
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 2 steps
1.1: diphenyl phosphoryl azide; triethylamine / 6 h / 90 °C / Inert atmosphere
2.1: N,N-dimethyl-formamide / 42 h / 80 °C / Inert atmosphere
2.2: 0.25 h / 0 °C
With diphenyl phosphoryl azide; triethylamine; In N,N-dimethyl-formamide;
|

methyl 2-amino-5-bromo-pyridine-4-carboxylate

C20H19BrFN3O4
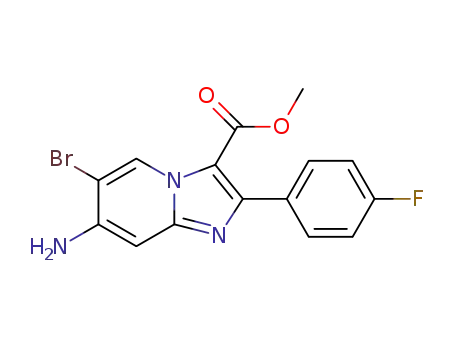
C15H11BrFN3O2
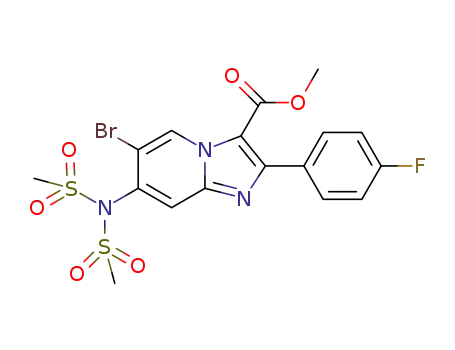
C17H15BrFN3O6S2
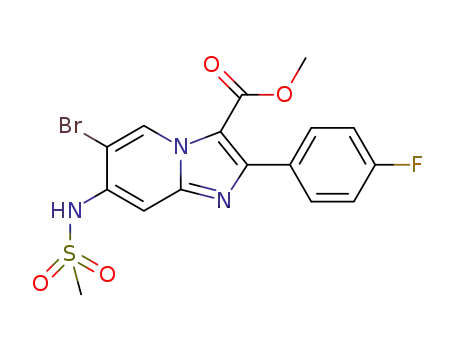
C16H13BrFN3O4S
CAS:56-94-0
CAS:1000018-10-9
CAS:866924-39-2