- +86-0533-2185556
- WhatsApp: +86 15965530500
- admin@hangyubiotech.com
Your Location:Home >Products >small-molecule inhibitor >520-18-3
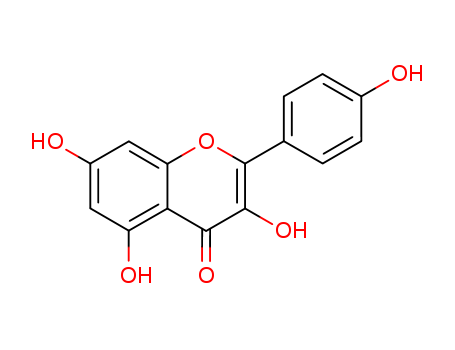

pd_meltingpoint:276 °C
Appearance:Yellow solid
Purity:99%
|
Pharmacological effects |
Kaempferol (3,5,7‐trihydroxy‐2‐[4‐hydroxyphenyl]‐4H‐1‐benzopyran‐4‐one) is a yellow bioactive flavonoid, which is present inmany edible plants such as tea, cabbage, broccoli, endive, kale, beans, tomato, strawberries, leek, and grapes. It has a significant role in reducing cancer and can act as a therapeutic agent in the treatment of diseases and ailments such as diabetes, obesity, cardiovascular diseases, oxidative stress, asthma, and microbial contamination disorders. Its efficacy, a broad range of activity, and low toxicity compared with other examined compounds, make it an attractive chemical in the fight against diseases (including cancer). |
|
Mechanism of action |
Kaempferol acts through different mechanisms: It induces apoptosis (HeLa cervical cancer cells), decreases cell viability (G2/M phase), downregulates phosphoinositide 3‐kinase (PI3K)/AKT (protein kinase B) and human T‐cell leukemia/lymphoma virus‐I (HTLV-I) signaling pathways, suppresses protein expression of epithelial‐mesenchymal transition (EMT)‐related markers including N‐cadherin, E‐cadherin, Slug, and Snail, and metastasis‐related markers such as matrix metallopeptidase 2 (MMP-2). |
|
Bioactivity |
As an anti‐oxidant, kaempferol counteracts production of superoxide ions and lowers the formation of reactive oxygen and nitrogen species. It also scavenges Fenton‐generated hydroxyl radical, peroxynitrite, and hydroxyl radicals. Furthermore, kaempferol suppresses the activity of xanthine oxidase and enhances the activities of catalase, heme oxygenase‐1, and superoxide dismutase. |
|
Biological Activity |
Naturally occurring flavonoid found in Gingko biloba and red wines that activates the mitochondrial Ca 2+ uniporter (EC 50 = 7 μ M). Induces caspase-9-mediated apoptosis in a variety of cancer cell lines via downregulation of polo-like kinase 1 (PLK1) expression. Exhibits antioxidant activity and attenuates osteoclastic bone reabsorption in vitro . |
|
Biochem/physiol Actions |
Potent inhibitor of osteoclastic bone resorption. The effect is believed to be attributable to both the antioxidant and estrogenic activities of kaempferol. |
|
Anticancer Research |
Kaempferol is one of the secondary metabolites found in some plants, plant-derivedfoods, and traditional medicines. It is a flavonoid compound obtained from someedible plants including grapes, tea, strawberries, broccoli, tomato, cabbage, leek,kale, endive, and beans. It inhibits growth and migration of pancreatic cancer cellsby acting on proto-oncogene tyrosine kinase (Src), ERK1/2, and AKT pathways(Singh et al. 2016a). It is being investigated in pancreatic and lung cancers toevaluate its antiangiogenic, anticancer, and radical scavenging activities. It showsmoderate cytostatic activity in PC3, HeLa, and K562 human cancer cells. It isidentified as aryl hydrocarbon receptor antagonist and acts against ABCG2 (ATP-bindingcassette subfamily G member 2)-mediated multidrug resistance bypreventing the ABCG2 upregulation in esophageal carcinoma. It induces theapoptosis of ovarian cancer cell by activating p53 in intrinsic pathway mechanism.It is an inhibitor of breast cancer resistance protein (BCRP), quinine reductase-2,and a substrate of BCRP (Calderon-Montano et al. 2011; Wang et al. 2012). |
|
General Description |
Kaempferol is a flavonol compound with the chemical name 3,4',5,7-tetrahydroxyflavone, also known by various synonyms such as rhamnolutein, robigenin, and trifolitin. It has been identified in plant sources like *Chenopodium ambrosioides* as both a free aglycone and in glycosylated forms (e.g., kaempferol 3-rhamnoside-4’-xyloside). Additionally, kaempferol serves as a key precursor in the semi-synthesis of bioactive compounds like icariin, where selective methylation and rearrangement reactions are employed to modify its structure. Its presence in diverse plant species underscores its chemotaxonomic and pharmacological significance. |
InChI:InChI=1/C15H10O6/c16-7-1-2-9(10(18)3-7)13-6-12(20)15-11(19)4-8(17)5-14(15)21-13/h1-6,16-19H
Inactivation of soybean urease in aqueou...
Kaempferol (2) was isolated from the aer...
-
-
-
-
Investigation of the constituents of Phy...
-
-
-
A new acylated flavonol glycoside, kaemp...
-
-
-
-
-
As part of the search for anticomplement...
Kaempferol (kae) and its glycosides are ...
-
Different chromatographic methods includ...
The composition of flavonoids from leave...
The synthesis of two series of five kaem...
Hippophae rhamnoides subsp. Sinensis is ...
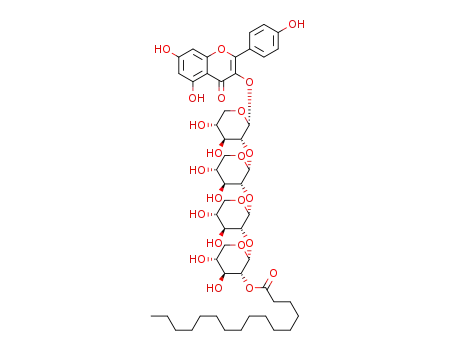
kaempferol-3-O-α-D-xylopyranosyl-(2a→1b)-2a-O-α-D-xylopyranosyl-(2b→1c)-2b-O-α-D-xylopyranosyl-(2c→1d)-2c-O-α-D-xylopyranosyl-2d-hexadecanoate

D-xylose

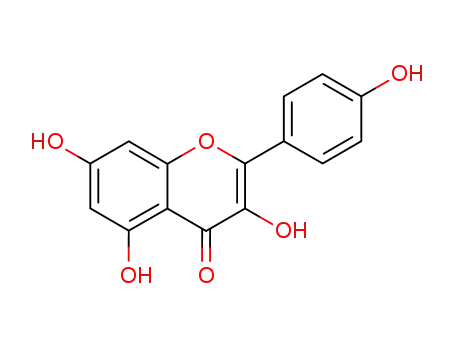
kaempferol

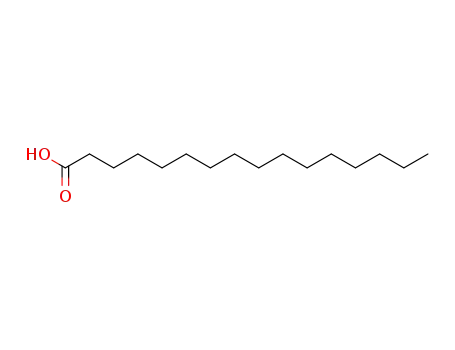
1-hexadecylcarboxylic acid
| Conditions | Yield |
|---|---|
|
kaempferol-3-O-α-D-xylopyranosyl-(2a→1b)-2a-O-α-D-xylopyranosyl-(2b→1c)-2b-O-α-D-xylopyranosyl-(2c→1d)-2c-O-α-D-xylopyranosyl-2d-hexadecanoate;
With
hydrogenchloride;
In
ethanol; water;
at 70 - 80 ℃;
With
hydrogenchloride;
In
ethanol; water;
|
C27H29O16(1-)*Na(1+)

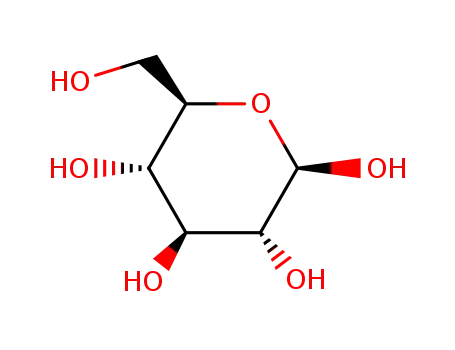
β-D-glucose


kaempferol
| Conditions | Yield |
|---|---|
|
With
hydrogenchloride;
In
water;
at 90 ℃;
for 5h;
|
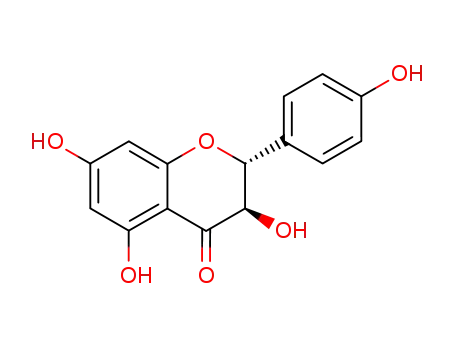
Aromadendrin
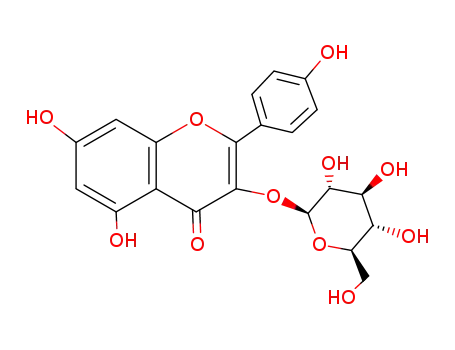
astragalin
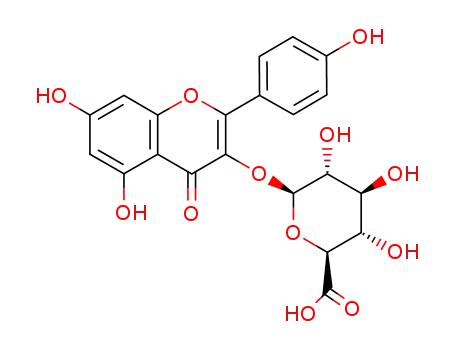
kaempferol 3-O-β-D-glucuronide
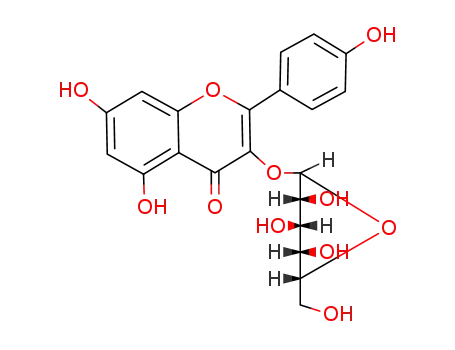
astragalin
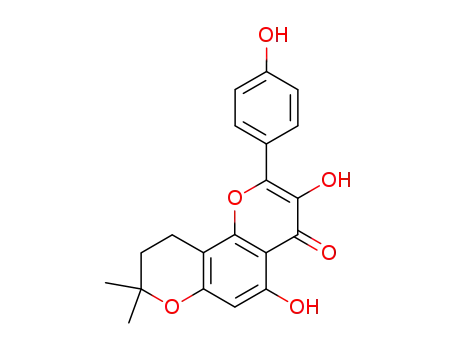
6,6-dimethyldihydropyran (2,3:7,8)-5,4'-dihydroxyflavonol
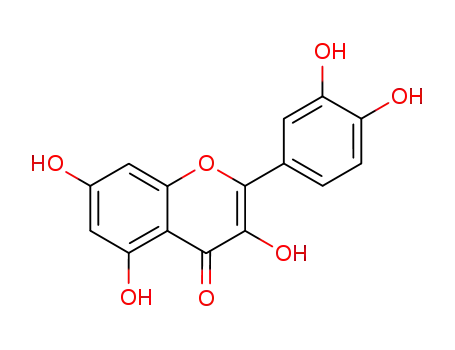
quercetol
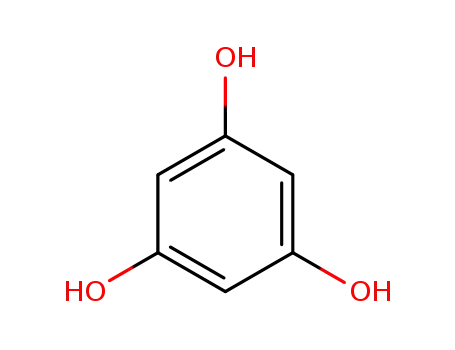
3,5-dihydroxyphenol
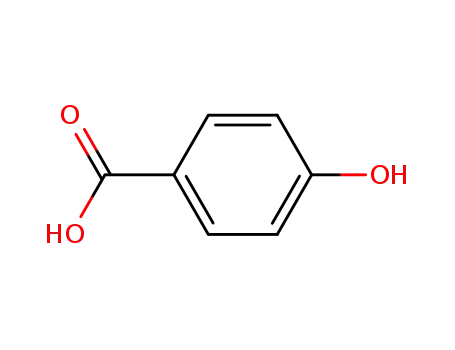
4-hydroxy-benzoic acid
CAS:112163-33-4
CAS:112-34-5
CAS:2781-11-5