- +86-0533-2185556
- WhatsApp: +86 15965530500
- admin@hangyubiotech.com
Your Location:Home >Products >small-molecule inhibitor >1693758-51-8

Purity:99%
Solid forms of chemical compounds that m...
Optimization of isoquinolinone PI3K inhi...
Compounds and pharmaceutical composition...
Compounds and pharmaceutical composition...
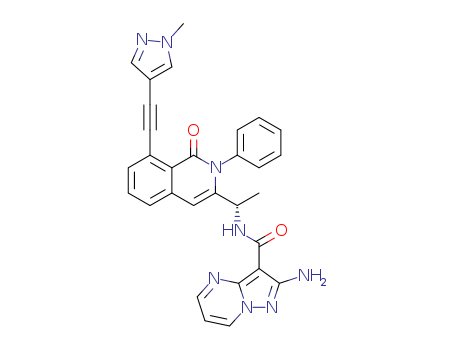
C23H20N4O

![2,5-dioxopyrrolidin-1-yl-2-aminopyrazolo[1,5-a]pyrimidine-3-carboxylate](/upload/2025/4/b42f67d1-a371-4637-b806-74e96413710d.png)
2,5-dioxopyrrolidin-1-yl-2-aminopyrazolo[1,5-a]pyrimidine-3-carboxylate

![(S)-2-amino-N-(1-(8-((1-methyl-1H-pyrazol-4-yl)ethynyl)-1-oxo-2-phenyl-1,2-dihydroisoquinolin-3-yl)ethyl)pyrazolo[1,5-a]pyrimidine-3-carboxamide](/upload/2025/4/bfecd096-5be4-40da-93d1-3b816c30aef3.png)
(S)-2-amino-N-(1-(8-((1-methyl-1H-pyrazol-4-yl)ethynyl)-1-oxo-2-phenyl-1,2-dihydroisoquinolin-3-yl)ethyl)pyrazolo[1,5-a]pyrimidine-3-carboxamide
| Conditions | Yield |
|---|---|
|
With
N-ethyl-N,N-diisopropylamine;
In
acetonitrile;
at 60 ℃;
Temperature;
Solvent;
Reagent/catalyst;
Inert atmosphere;
|
85% |
![2-aminopyrazolo[1,5-a]pyrimidine-3-carboxylic acid](/upload/2025/4/a16430b2-7e0a-431c-80f4-7791ccbc5392.png)
2-aminopyrazolo[1,5-a]pyrimidine-3-carboxylic acid

C23H20N4O

![(S)-2-amino-N-(1-(8-((1-methyl-1H-pyrazol-4-yl)ethynyl)-1-oxo-2-phenyl-1,2-dihydroisoquinolin-3-yl)ethyl)pyrazolo[1,5-a]pyrimidine-3-carboxamide](/upload/2025/4/bfecd096-5be4-40da-93d1-3b816c30aef3.png)
(S)-2-amino-N-(1-(8-((1-methyl-1H-pyrazol-4-yl)ethynyl)-1-oxo-2-phenyl-1,2-dihydroisoquinolin-3-yl)ethyl)pyrazolo[1,5-a]pyrimidine-3-carboxamide
| Conditions | Yield |
|---|---|
|
With
benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; N-ethyl-N,N-diisopropylamine;
In
N,N-dimethyl-formamide;
at 30 ℃;
for 16h;
Reagent/catalyst;
Solvent;
Temperature;
Inert atmosphere;
|
70% |
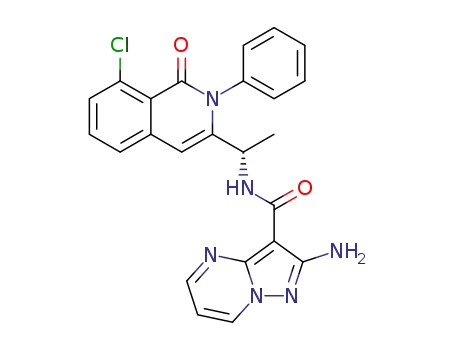
C24H19ClN6O2
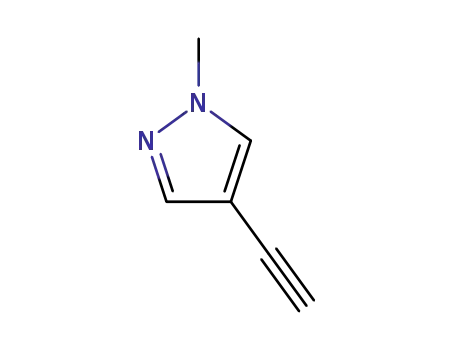
4-ethynyl-1-methyl-1-H-pyrazole
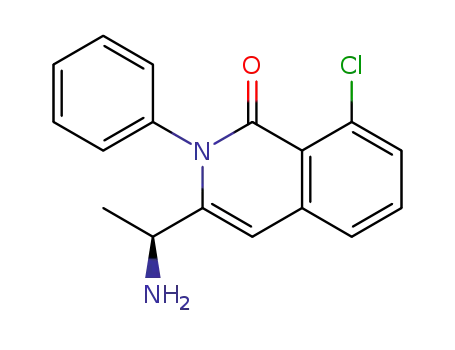
(S)-3-(1-aminoethyl)-8-chloro-2-phenylisoquinoline-1(2H)-one
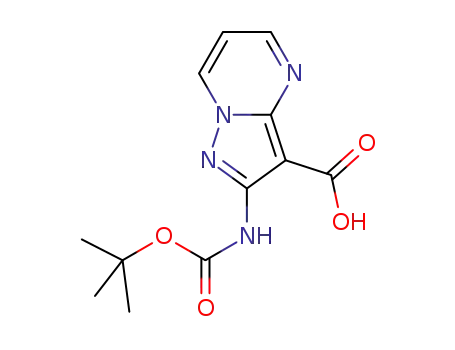
2-((tert-butoxycarbonyl)amino)pyrazolo[1,5-a]pyrimidine-3-carboxylic acid
CAS:112163-33-4
CAS:112-84-5
CAS:86347-15-1
CAS:864731-61-3