- +86-0533-2185556
- WhatsApp: +86 15965530500
- admin@hangyubiotech.com
Your Location:Home >Products >Pharmaceutical intermediate >103-49-1


pd_meltingpoint:-26 °C(lit.)
Appearance:Colorless to light yellow liquid
Purity:99%
|
Preparation |
Dibenzylamine is obtained by the reaction of benzyl chloride and liquid ammonia in ethanol. |
|
Synthesis Reference(s) |
Tetrahedron Letters, 26, p. 4633, 1985 DOI: 10.1016/S0040-4039(00)98771-9The Journal of Organic Chemistry, 60, p. 5969, 1995 DOI: 10.1021/jo00123a041 |
|
Purification Methods |
Purify the amine by distillation in a vacuum. It causes burns to the skin. The dihydrochloride has m 265-266o (from MeOH/HCl), and the tetraphenyl boronate has m 129-133o. [Bradley & Maisey J Chem Soc 247 1954, Hall J Phys Chem 60 63 1956, Donetti & Bellora J Org Chem 37 3352 1972, Beilstein 12 IV 2179.] |
|
General Description |
Dibenzylamine (DBA) is a secondary amine used as a reactant in various synthetic processes, including the synthesis of 2-substituted 2H-chromenes, where it facilitates the reaction between potassium vinyltrifluoroborates and salicylaldehyde. It also serves as a substrate in oxidative N-dealkylation studies involving cupric hydroperoxide intermediates, leading to benzaldehyde formation. Additionally, dibenzylamine-derived compounds are employed in enantioselective reactions, such as the catalytic α-aminomethylation of aldehydes and dynamic kinetic resolution of α-halo acids, yielding β-dibenzylamino alcohols and other valuable intermediates for pharmaceuticals. Its versatility in organic synthesis highlights its role as a key reagent in producing heterocyclic compounds and chiral building blocks. |
|
Application |
Dibenzylamine is an important organic synthesis intermediate, mainly used to produce efficient and non-toxic vulcanization accelerators tetrabenzylthiuramdisulfide (TBZTD) and zinc dibenzyldithiocarbamate (ZBEC). Synthesize penicillin and curing agent for rubber and plastic curing, and can be used in the detection of cobalt, cyanate, and iron. |
InChI:InChI=1/C14H15N/c1-3-7-13(8-4-1)11-15-12-14-9-5-2-6-10-14/h1-10,15H,11-12H2/p+1
A new ionic liquid is presented as a med...
The integration of electro and bio-catal...
A comprehensive methodology to prepare n...
A one-pot reductive amination of aldehyd...
Benzothiazolesulfonamides of primary and...
Developing more efficient routes to achi...
A novel and highly efficient heterogeneo...
Imines have been reduced to amines in hi...
Two iridium(III) complexes containing a ...
A series of well-defined iron(II) comple...
The copper-catalyzed intermolecular enan...
-
Lithium triethylborohydride has been fou...
A series of thioureas containing two aro...
An earth-abundant metal cobalt catalyst ...
Deoxygenative hydrogenation of amides to...
A specific secondary phosphine oxide (SP...
(Chemical Equation Presented) Direct tra...
-
Copper oxide catalysts have been prepare...
An unprecedented photocatalytic system c...
By applying a simple Pd/NiO catalyst, th...
Described herein is that the selective r...
The reactions of a new type of quinonoid...
A simple and efficient method for the cl...
Quaternary ammonium salts improve the so...
De novo syntheses of amides often genera...
A ketoester resin was developed as the b...
A series of iridium and rhodium complexe...
Deprotection of N-benzenesulfonamides or...
A facile process for the catalyst-free a...
-
-
formula presented A broad range of benza...
Based on the hemilability, a novel N-het...
A γ-Al2O3 supported Ni and Cu bimetallic...
The synthesis and characterization of ai...
Different titanocene complexes 1-10 were...
In this paper, we describe the catalytic...
We have developed a mild, convenient, an...
A modified process using inexpensive poi...
The reaction of N,N-disubstituted hydrox...
An efficient and highly chemoselective s...
Silver nanoparticles supported on alumin...
-
Reactions of (2-(pyren-1-ylmethylene)hyd...
Trichlorosilane-dimethylformamide (Cl3Si...
A comparative study of the performance o...
A visible light promoted process for des...
Efficient SO3H-functionalized metal orga...
Tandem iron/zinc or iron/indium-catalyse...
Treatment of salicylaldiminato ligand L1...
A terminal [Ni-OH] complex1, supported b...
The metal-free catalytic hydrogenation o...

N-benzylidene benzylamine

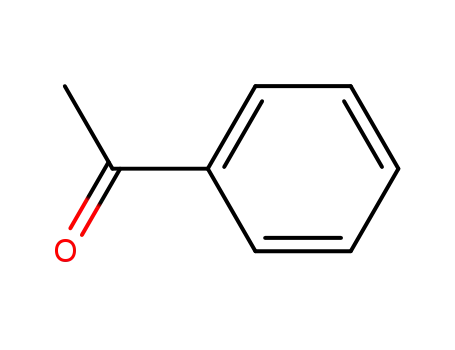
acetophenone

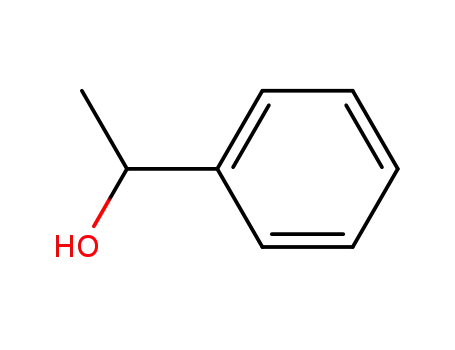
1-Phenylethanol


dibenzylamine
| Conditions | Yield |
|---|---|
|
With
tetrakis[3,5-bis(trifluoromethyl)phenyl]boric acid bis(diethyl ether) complex; C32H63CoNP2Si; hydrogen;
In
tetrahydrofuran;
at 25 ℃;
for 21h;
under 760.051 Torr;
|
40 %Chromat. |
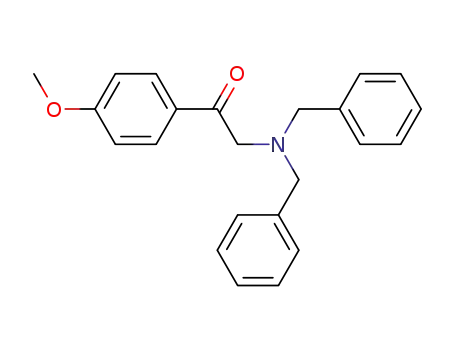
2-Dibenzylamino-1-(4-methoxy-phenyl)-ethanone

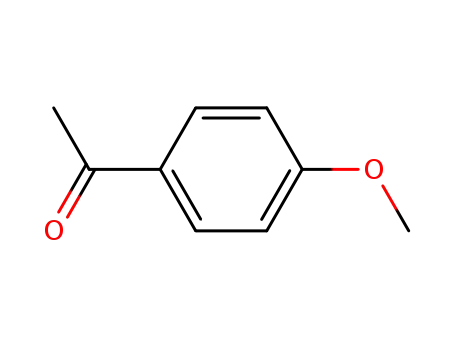
1-(4-methoxyphenyl)ethanone


dibenzylamine
| Conditions | Yield |
|---|---|
|
With
acetic acid; zinc;
at 20 ℃;
for 1h;
|
90% 84% |
tetrachloromethane
N,N'-bis(dibenzylamino)diazene
formaldehyd
N,N-dibenzylaniline
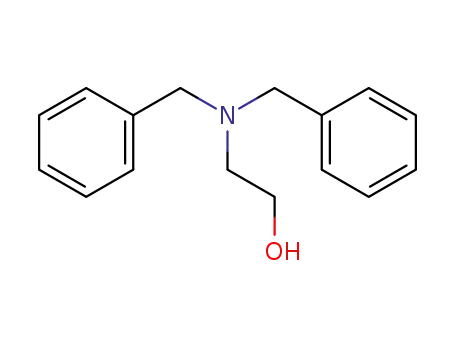
N,N-dibenzyl-2-aminoethanol
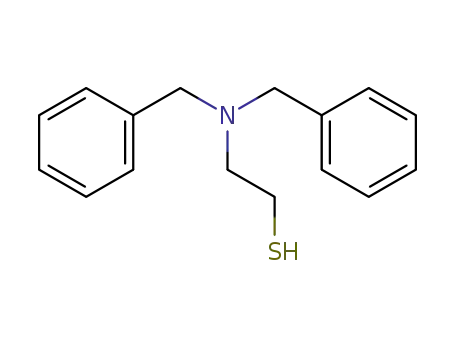
2-dibenzylamino-ethanethiol
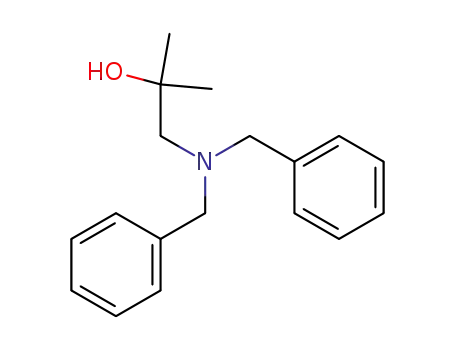
1-dibenzylamino-2-methyl-propan-2-ol
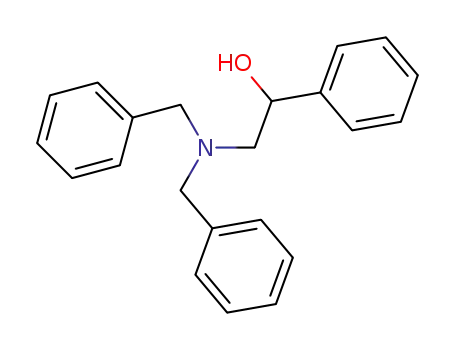
(+/-)-dibenzyl(β-hydroxyphenethyl)amine
CAS:112163-33-4
CAS:112-84-5
CAS:864731-61-3