- +86-0533-2185556
- WhatsApp: +86 15965530500
- admin@hangyubiotech.com
Your Location:Home >Products >API >1632051-40-1

Purity:99%
This invention relates to a 19-nor C3,3-...
Certain classes of neuroactive steroids ...
Provided herein are 19-nor C3,3-disubsti...
![2-bromo-1-((3R,5R,8R,9R,10S,13S,14S,17S)-3-hydroxy-3,13-dimethylhexadecahydro-1H-cyclopenta[a]phenanthren-17-yl)ethan-1-one](/upload/2025/4/5e68d3ea-ef9c-43f4-bbb4-3341619d100b.png)
2-bromo-1-((3R,5R,8R,9R,10S,13S,14S,17S)-3-hydroxy-3,13-dimethylhexadecahydro-1H-cyclopenta[a]phenanthren-17-yl)ethan-1-one

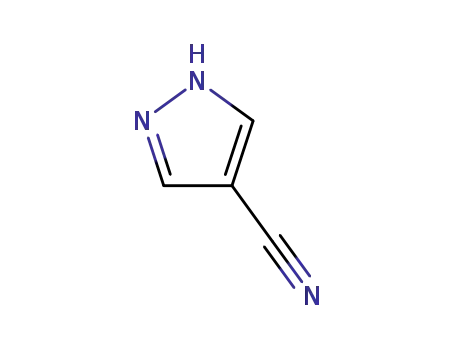
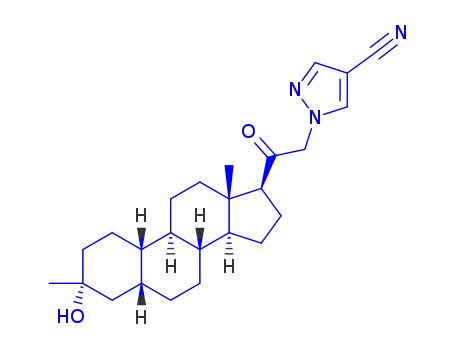
1H-pyrazole-4-carbonitrile

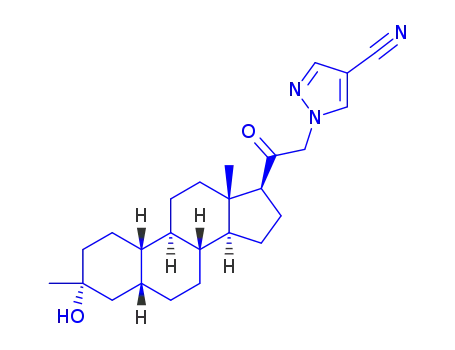
zuranolone
| Conditions | Yield |
|---|---|
|
With potassium carbonate; In tetrahydrofuran; at 20 ℃; for 15h;
|
17.4% |
|
With potassium carbonate; In tetrahydrofuran; at 20 ℃; for 15h;
|
17.4% |
|
With potassium carbonate; In tetrahydrofuran; at 20 ℃; for 15h;
|
17.4% |
|
With potassium carbonate; In tetrahydrofuran;
|
28 g |
![(3R,5R,8R,9R,10S,13S,14S)-3-hydroxy-3,13-dimethyl-1,2,4,5,6,7,8,9,10,1 1,12,14,15,16-tetradecahydrocyclopenta[a]phenanthren-17-one](/upload/2025/4/ad504974-877b-43fb-80f7-7e2568953c20.png)
(3R,5R,8R,9R,10S,13S,14S)-3-hydroxy-3,13-dimethyl-1,2,4,5,6,7,8,9,10,1 1,12,14,15,16-tetradecahydrocyclopenta[a]phenanthren-17-one


zuranolone
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 5 steps
1.1: potassium tert-butylate / tetrahydrofuran / 1 h / 0 - 60 °C
1.2: 18 h / 60 °C
2.1: dimethylsulfide borane complex / tetrahydrofuran / 14 - 20 °C / Cooling with ice
2.2: 2 h / 14 - 20 °C
3.1: pyridinium chlorochromate / dichloromethane / 3 h / 0 - 22 °C
4.1: bromine; hydrogen bromide / water; methanol / 1.5 h / 17 °C
5.1: potassium carbonate / tetrahydrofuran / 15 h / 20 °C
With dimethylsulfide borane complex; potassium tert-butylate; hydrogen bromide; bromine; potassium carbonate; pyridinium chlorochromate; In tetrahydrofuran; methanol; dichloromethane; water;
|
|
|
Multi-step reaction with 5 steps
1: potassium tert-butylate / tetrahydrofuran
2: borane / tetrahydrofuran
3: pyridinium chlorochromate / dichloromethane
4: bromine; hydrogen bromide / water; methanol
5: potassium carbonate / tetrahydrofuran
With borane; potassium tert-butylate; hydrogen bromide; bromine; potassium carbonate; pyridinium chlorochromate; In tetrahydrofuran; methanol; dichloromethane; water;
|
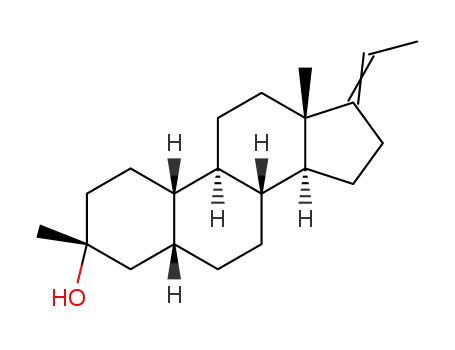
(3R,5R,8R,9R,10S,13S,14S)-17-ethylidene-3,13-dimethylhexadecahydro-1H-cyclopenta[a]phenanthren-3-ol
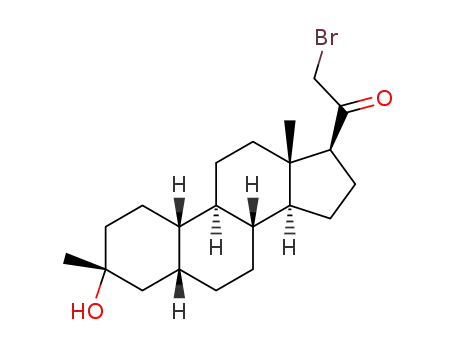
2-bromo-1-((3R,5R,8R,9R,10S,13S,14S,17S)-3-hydroxy-3,13-dimethylhexadecahydro-1H-cyclopenta[a]phenanthren-17-yl)ethan-1-one

1H-pyrazole-4-carbonitrile
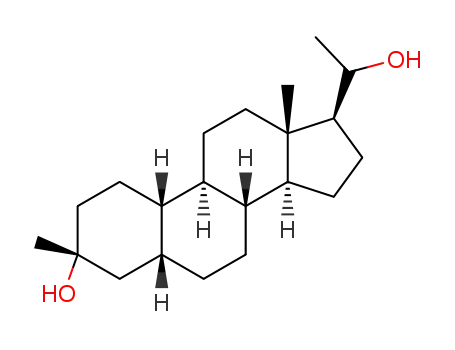
(3R,5R,8R,9R,10S,13S,14S,17S)-17-(1-hydroxyethyl)-3,13-dimethylhexadecahydro-1H-cyclopenta[a]phenanthren-3-ol
CAS:52190-28-0
CAS:4733-39-5
CAS:2093152-77-1
CAS:196929-78-9