- +86-0533-2185556
- WhatsApp: +86 15965530500
- admin@hangyubiotech.com
Your Location:Home >Products >API >196929-78-9

pd_meltingpoint:103-107 °C(lit.)
Appearance:white to light yellow crystal powder
Purity:99%
InChI:InChI=1/C6H17NS.H2O/c1-6(2,3)8(4,5)7;/h7H2,1-5H3;1H2
(Matrix presented) An improved synthesis...
The first example of the catalytic asymm...
The invention belongs to the technical f...
The invention discloses a method of synt...
The invention discloses a method for pre...
The invention discloses a method for pre...
![2-Methyl-propane-2-sulfinic acid ((R)-3-[1,3]dioxan-2-yl-1-phenyl-propyl)-amide](/upload/2025/4/572f7dd0-1dd1-4ff6-8fe3-35aadee6fd6d.png)
2-Methyl-propane-2-sulfinic acid ((R)-3-[1,3]dioxan-2-yl-1-phenyl-propyl)-amide

2-phenyl-3,4-dihydro-2H-pyrrole

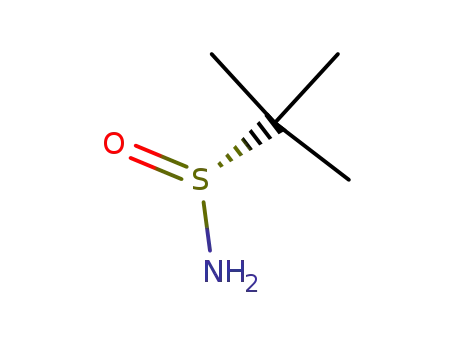
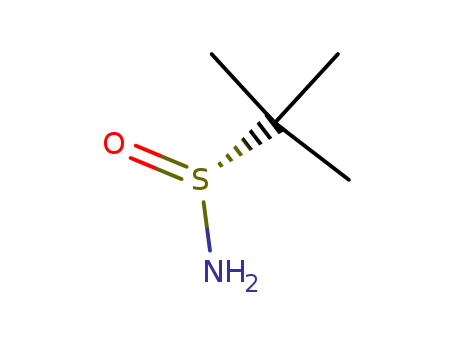
(R)-2-methylpropane-2-sulfinamide

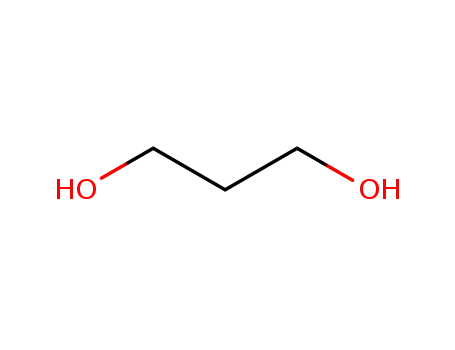
trimethyleneglycol
| Conditions | Yield |
|---|---|
|
With trifluoroacetic acid-d1; In water-d2; for 0.25h;
|
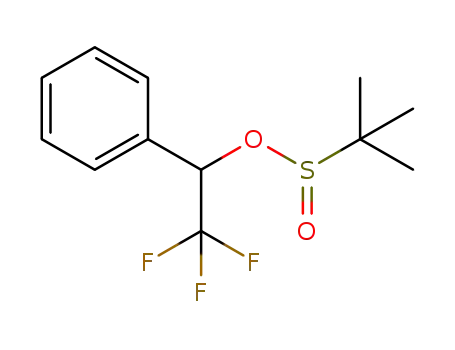
2,2,2-trifluoro-1-phenylethyl 2-methylpropane-2-sulfinate

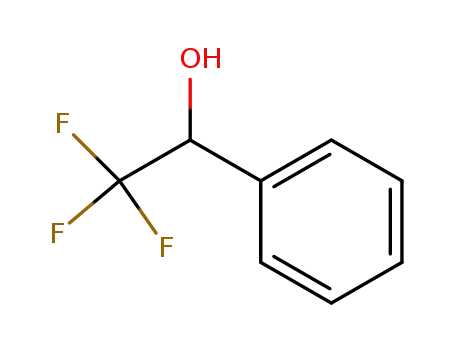
1-phenyl-2,2,2-trifluoromethylethanol


(R)-2-methylpropane-2-sulfinamide
| Conditions | Yield |
|---|---|
|
With lithium hexamethyldisilazane; In tetrahydrofuran; at 0 - 20 ℃; for 10h; Inert atmosphere;
|

(R)-tert-butyl tert-butanethiosulfinate
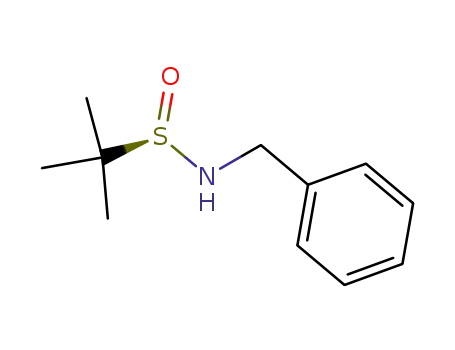
(RS)-N-benzyl-2-methylpropane-2-sulfinamide
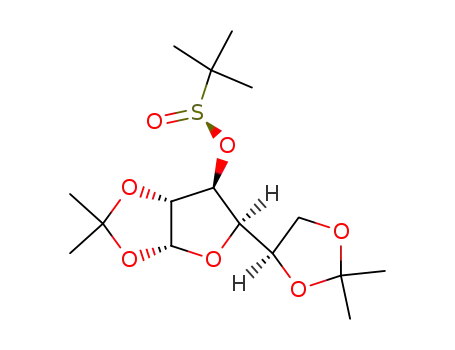
(R)-3-deoxy-1,2:5,6-di-O-isopropylidene-α-D-glucofuranos-3-yl tert-butanesulfinate
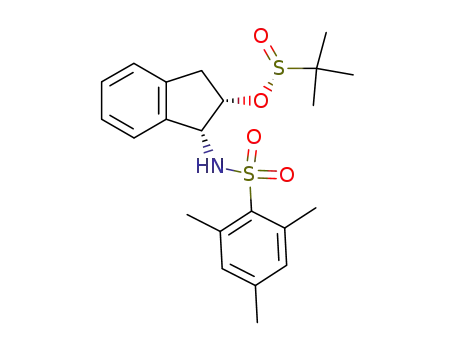
2-Methyl-propane-2-sulfinic acid (1R,2S)-1-(2,4,6-trimethyl-benzenesulfonylamino)-indan-2-yl ester
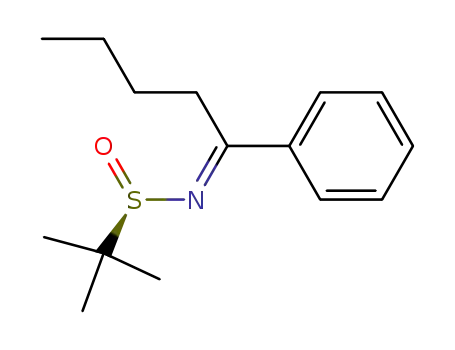
(RS)-2-methyl-N-(1-phenylpentylidene)propane-2-sulfinamide
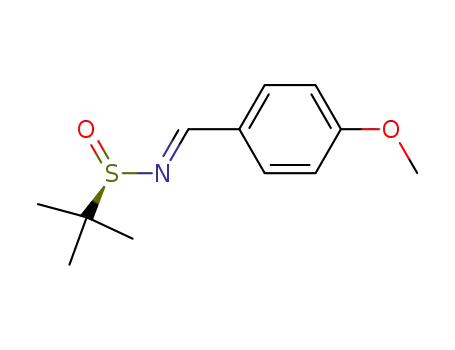
(R,E)-N-(4-methoxybenzylidene)-2-methylpropane-2-sulfinamide
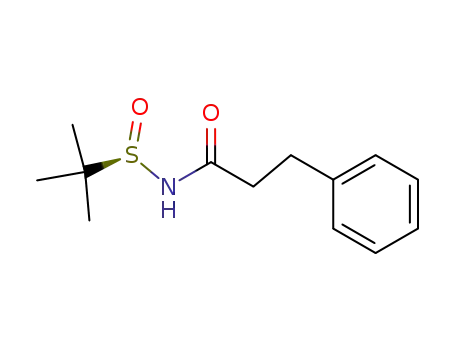
(R)-N-hydrocinnamoyl-2-methyl-2-propanesulfinamide
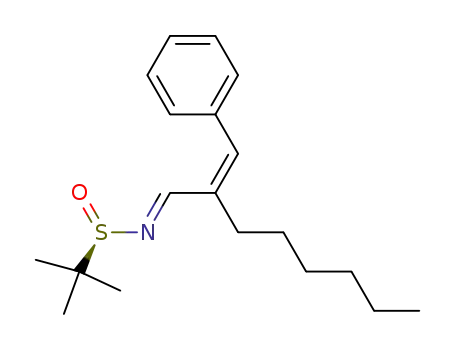
(R)-(-)-N-(2-hexyl-3-phenyl-allylidene)-2-methylpropanesulfinamide
CAS:115473-15-9
CAS:1173-88-2
CAS:1632051-40-1
CAS:2182601-74-5