- +86-0533-2185556
- WhatsApp: +86 15965530500
- admin@hangyubiotech.com
Your Location:Home >Products >API >16603-18-2
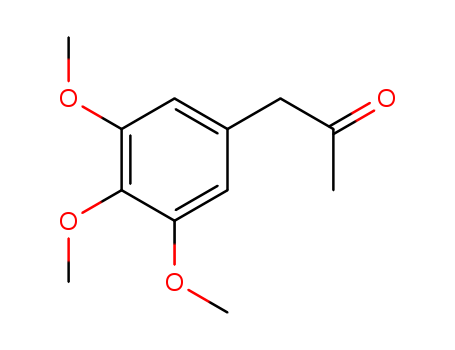

pd_meltingpoint:58-62°C
Purity:99%
|
General Description |
3,4,5-Trimethoxyphenylacetone is a chemical compound with a molecular formula of C12H16O4. It is a phenylacetone derivative and is also known by the chemical name "Vanillin Acetate". 3,4,5-TRIMETHOXYPHENYLACETONE is a clear, colorless to yellowish liquid with a sweet, floral odor. It is commonly used as a flavoring agent in the food industry, particularly in the production of vanilla flavoring. Additionally, 3,4,5-trimethoxyphenylacetone has been studied for its potential antimicrobial and antioxidant properties. 3,4,5-TRIMETHOXYPHENYLACETONE is also used in the synthesis of various pharmaceuticals and other organic compounds. |
InChI:InChI=1/C12H16O4/c1-8(13)5-9-6-10(14-2)12(16-4)11(7-9)15-3/h6-7H,5H2,1-4H3
A convenient and efficient method was de...
An operationally simple and mild one-pot...
Mor(DalPhos) for Me(sylates): Described ...
Set the ace(tone): A palladium catalyst ...
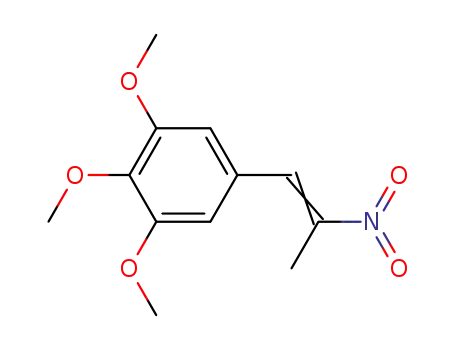
1-(3,4,5-trimethoxyphenyl)-2-nitro-1-propene

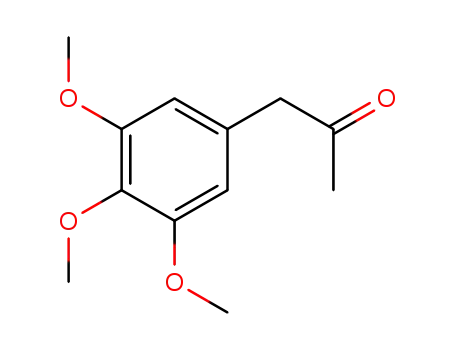
(3,4,5-trimethoxyphenyl)acetone
| Conditions | Yield |
|---|---|
|
With hydrogen; acetic acid; nickel; at 25 ℃; for 15h;
|
65% |
|
With hydrogenchloride; iron(III) chloride; iron;
|
|
|
With hydrogenchloride; iron(III) chloride; iron; In toluene; Heating;
|
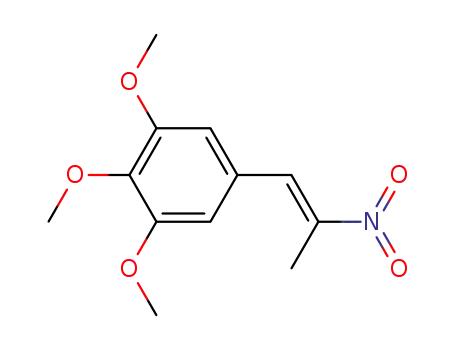
(E)-1,2,3-trimethoxy-5-(2-nitroprop-1-enyl)benzene


(3,4,5-trimethoxyphenyl)acetone
| Conditions | Yield |
|---|---|
|
With iron; In acetic acid; at 20 ℃; for 0.5h;
|
94% |

1-(3,4,5-trimethoxyphenyl)-2-nitro-1-propene
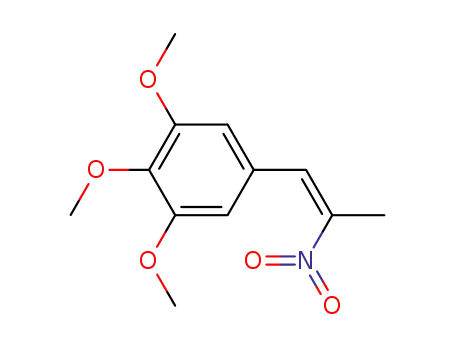
1,2,3-trimethoxy-5-(2-nitro-propenyl)benzene
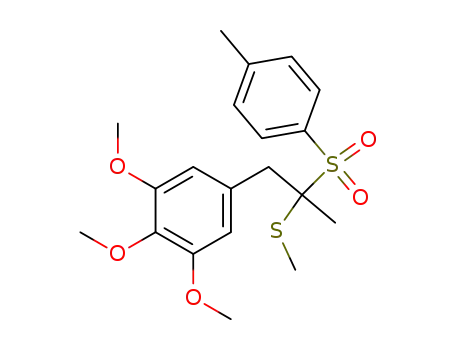
1,2,3-Trimethoxy-5-[2-methylsulfanyl-2-(toluene-4-sulfonyl)-propyl]-benzene
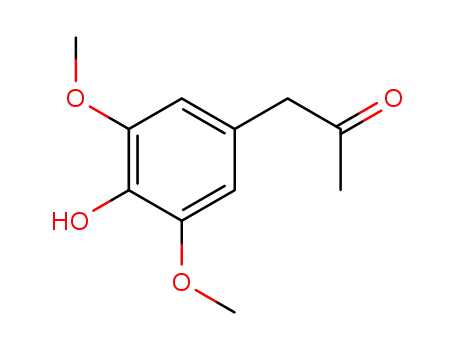
1-(3,5-dimethoxy-4-hydroxyphenyl)propan-2-one
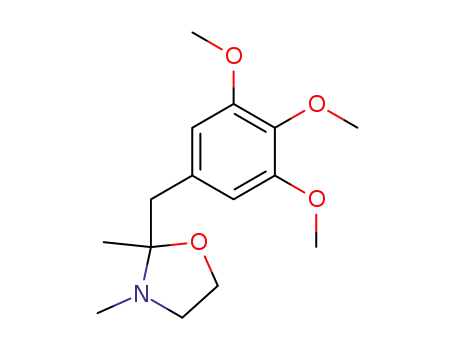
2-(3,4,5-trimethoxybenzyl)-2,3-dimethyloxazolidine
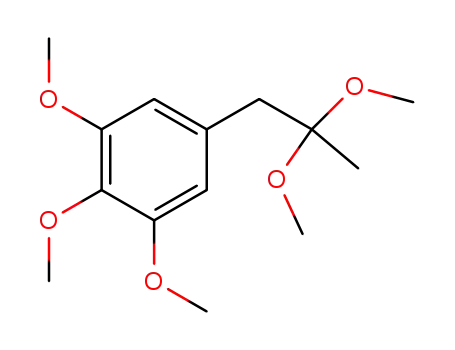
2,2-dimethoxy-1-(3,4,5-trimethoxyphenyl)propane
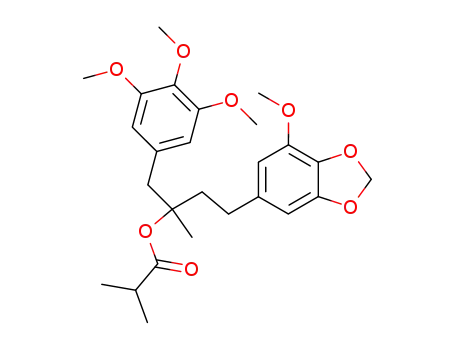
2-isopropylcarbonyloxy-4-(3-methoxy-4,5-methylenedioxyphenyl)-2-methyl-1-(3,4,5-trimethoxyphenyl)butane
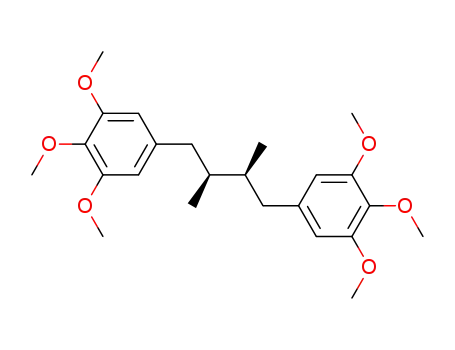
5,5'-(2,3-dimethylbutane-1,4-diyl)bis(1,2,3-trimethoxybenzene)
CAS:112163-33-4
CAS:112-34-5
CAS:2182601-74-5
CAS:364-98-7