- +86-0533-2185556
- WhatsApp: +86 15965530500
- admin@hangyubiotech.com
Your Location:Home >Products >small-molecule inhibitor >1321514-06-0
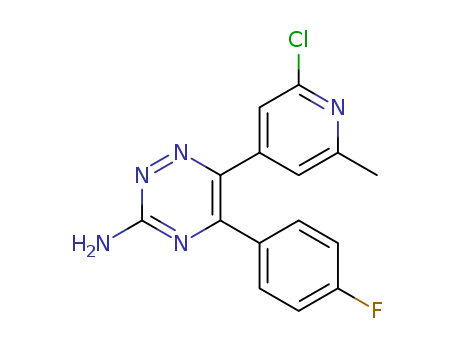

Purity:99%
The AstraZeneca approach to synthetic Ro...
An efficient route to AZD4635 has been d...
According to the invention there is prov...
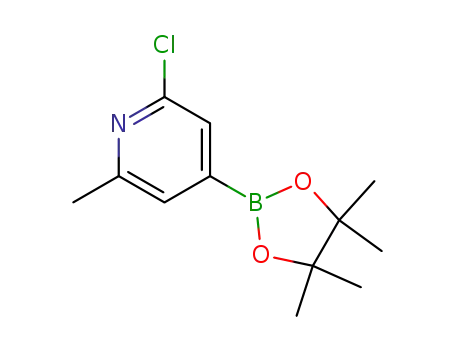
2-chloro-6-methyl-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)pyridine

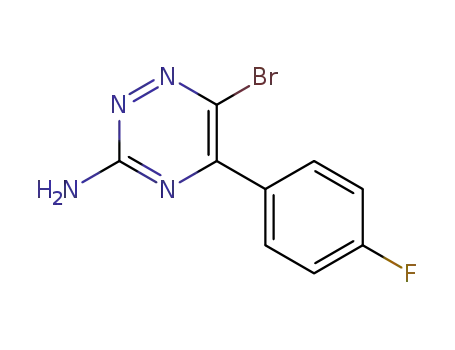
6-bromo-5-(4-fluorophenyl)-1,2,4-triazin-3-amine

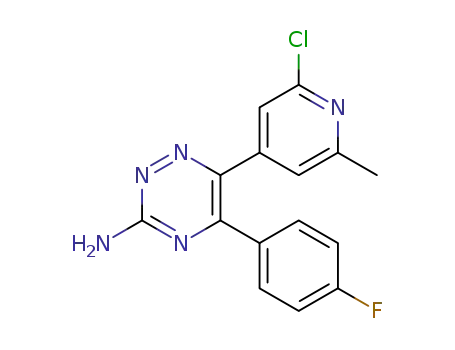
6-(2-chloro-6-methylpyridin-4-yl)-5-(4-fluorophenyl)-1,2,4-triazin-3-amine
| Conditions | Yield |
|---|---|
|
With
(4-(N,N-dimethylamino)phenyl)-di-tert-butylphosphine; palladium diacetate; potassium carbonate;
In
tetrahydrofuran; water;
at 62 - 66 ℃;
Inert atmosphere;
Large scale;
|
84.5% |
|
With
potassium carbonate;
dichloro bis((p-dimethylaminophenyl)-?-di-tert-butylphosphine)palladium(II);
In
1,4-dioxane; water;
Product distribution / selectivity;
Inert atmosphere;
Reflux;
|
61% |
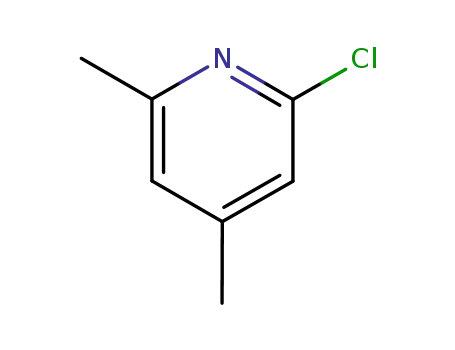
2-chloro-4,6-dimethyl-pyridine


6-(2-chloro-6-methylpyridin-4-yl)-5-(4-fluorophenyl)-1,2,4-triazin-3-amine

C15H11ClFN5
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 3 steps
1.1: lithium hexamethyldisilazane / tetrahydrofuran / 1 h / 0 °C / Inert atmosphere
1.2: 15 h / 20 °C / Inert atmosphere
2.1: trifluoroacetic acid / dichloromethane / 24 h / Inert atmosphere
3.1: zinc trifluoromethanesulfonate / isopropyl alcohol / 24 h / 50 °C
With
zinc trifluoromethanesulfonate; trifluoroacetic acid; lithium hexamethyldisilazane;
In
tetrahydrofuran; dichloromethane; isopropyl alcohol;
|
|
|
Multi-step reaction with 3 steps
1: lithium hexamethyldisilazane / tetrahydrofuran / 10 °C / Inert atmosphere
2: toluene-4-sulfonic acid; N-chloro-succinimide / water; dichloromethane / 2 h / Inert atmosphere
3: sodium iodide / ethanol / 20 - 75 °C / Inert atmosphere
With
N-chloro-succinimide; toluene-4-sulfonic acid; sodium iodide; lithium hexamethyldisilazane;
In
tetrahydrofuran; ethanol; dichloromethane; water;
|
|
|
Multi-step reaction with 3 steps
1: lithium hexamethyldisilazane / tetrahydrofuran / 10 °C / Inert atmosphere
2: copper(II) acetate monohydrate; potassium carbonate; 2,3-dimethyl-2,3-diaminobutane / acetonitrile / 72 h
3: zinc trifluoromethanesulfonate / isopropyl alcohol / 24 h / 50 °C
With
2,3-dimethyl-2,3-diaminobutane; copper(II) acetate monohydrate; zinc trifluoromethanesulfonate; potassium carbonate; lithium hexamethyldisilazane;
In
tetrahydrofuran; isopropyl alcohol; acetonitrile;
|
|
|
Multi-step reaction with 3 steps
1: lithium hexamethyldisilazane / 2-methyltetrahydrofuran / 20 °C / Inert atmosphere
2: toluene-4-sulfonic acid; N-chloro-succinimide / water; dichloromethane / 2 h / Inert atmosphere
3: sodium iodide / ethanol / 20 - 75 °C / Inert atmosphere
With
N-chloro-succinimide; toluene-4-sulfonic acid; sodium iodide; lithium hexamethyldisilazane;
In
2-methyltetrahydrofuran; ethanol; dichloromethane; water;
|
|
|
Multi-step reaction with 3 steps
1: lithium hexamethyldisilazane / 2-methyltetrahydrofuran / 20 °C / Inert atmosphere
2: copper(II) acetate monohydrate; potassium carbonate; 2,3-dimethyl-2,3-diaminobutane / acetonitrile / 72 h
3: zinc trifluoromethanesulfonate / isopropyl alcohol / 24 h / 50 °C
With
2,3-dimethyl-2,3-diaminobutane; copper(II) acetate monohydrate; zinc trifluoromethanesulfonate; potassium carbonate; lithium hexamethyldisilazane;
In
2-methyltetrahydrofuran; isopropyl alcohol; acetonitrile;
|

2-chloro-6-methyl-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)pyridine

6-bromo-5-(4-fluorophenyl)-1,2,4-triazin-3-amine
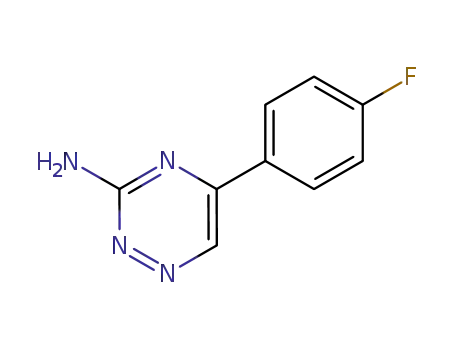
5-(4-fluorophenyl)-1,2,4-triazin-3-amine
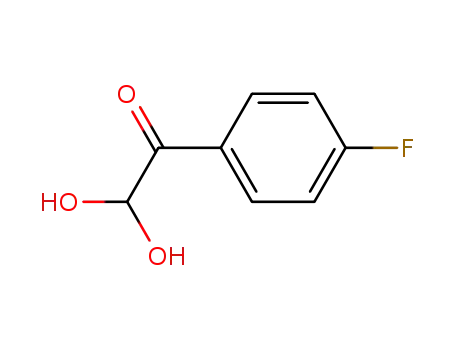
1-(4-fluorophenyl)-2,2-dihydroxyethan-1-one
CAS:109-15-9
CAS:30501-29-2
CAS:155206-00-1
CAS:1052207-59-6