- +86-0533-2185556
- WhatsApp: +86 15965530500
- admin@hangyubiotech.com
Your Location:Home >Products >small-molecule inhibitor >24979-70-2

pd_meltingpoint:360oC (dec.)
Purity:99%
|
General Description |
Poly(4-vinylphenol) (PVP) is a polymeric cross-linker mainly used as a layer to improve adhesion by forming a non-toxic and low cost film. It is an acidic polymer which consists of more than 100 hydroxyl groups in one molecule of PVP which result in high stability and complexation of the films. |
InChI:InChI=1/C8H8O/c1-2-7-3-5-8(9)6-4-7/h2-6,9H,1H2
4-Vinylphenols, useful compounds for ind...
-
Two flexible and colorless polymers are ...
Hydroxycinnamic acid (HCA) decarboxylati...
4-Vinylphenyl glycidyl ether (4VPGE), an...
To simply prepare hyperbranched polystyr...
A novel animation and 1,2-amino,hydro-el...
p-Coumaric acid (1) is an abundant pheno...
We present a new design for non-linear o...
-
It was previously reported that cell cul...
1H-Tetrazoles possess the lowest pKa wit...
The compounds 4-vinylphenol (4-VP), 4-vi...
An efficient synthesis of 4-vinylphenol ...
Continuous flow systems for chemical syn...
A new series of anionic photoacid genera...
The current trend for future flame retar...
The present invention provides a synthes...
The value of catalytic dehydrogenation o...
The vanillyl-alcohol oxidase (VAO) famil...
A modified monomer useful for the polyme...
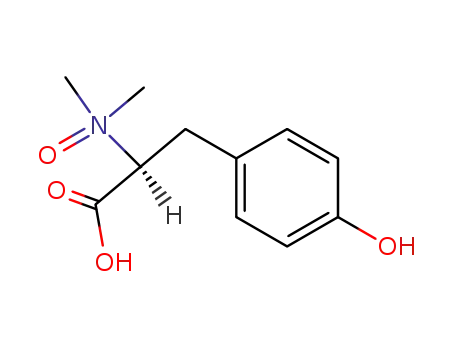
N,N-dimethyl-L-tyrosine N-oxide

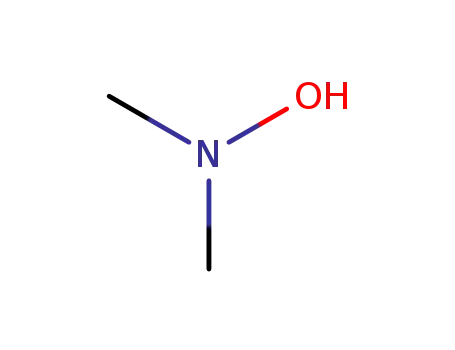
N,N-dimethylhydroxylamine

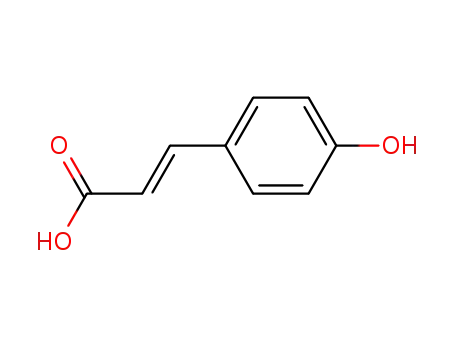
p-Coumaric Acid

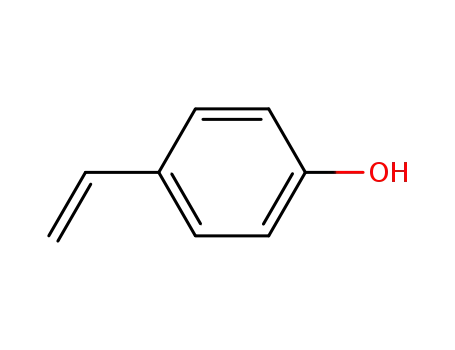
4-Vinylphenol
| Conditions | Yield |
|---|---|
|
In
N,N-dimethyl acetamide;
at 130 ℃;
Product distribution / selectivity;
|
75% 3.3% |
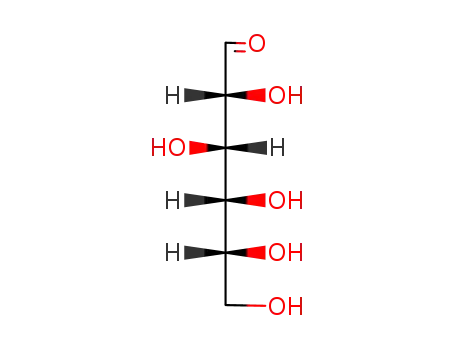
D-glucose

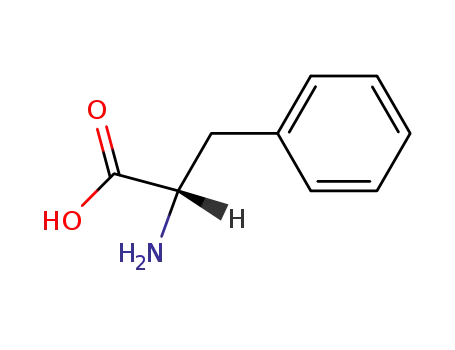
L-phenylalanine

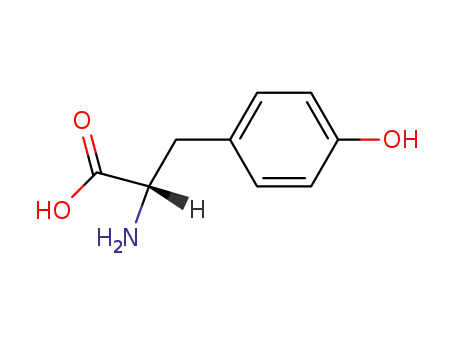
L-tyrosine


4-Vinylphenol

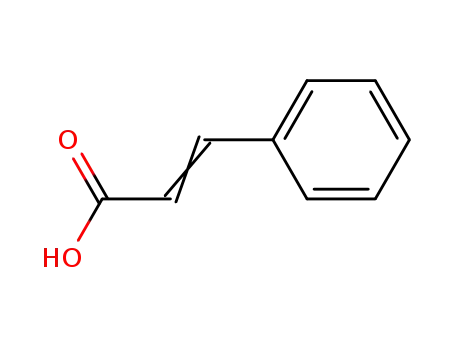
Cinnamic acid
| Conditions | Yield |
|---|---|
|
With
E. coli strain WWQ51.1;
at 35 ℃;
for 50h;
pH=6.8 - 7.0;
Microbiological reaction;
|
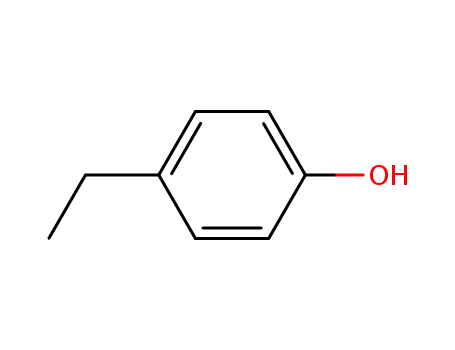
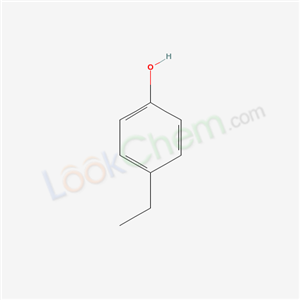
4-Ethylphenol
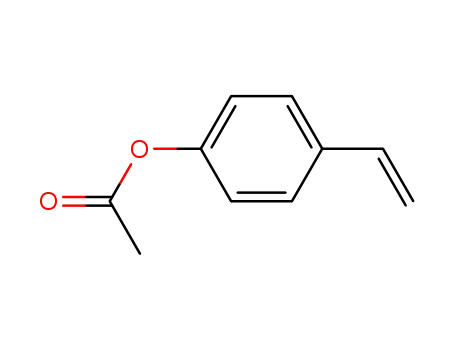
p-acetoxystyrene

p-Coumaric Acid
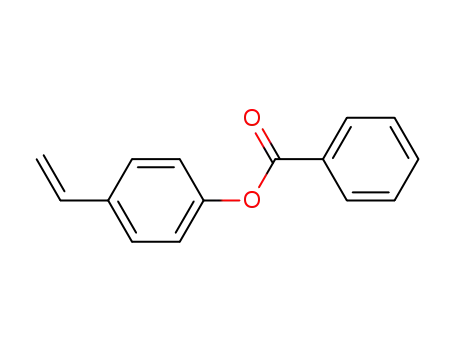
4-vinylphenyl benzoate

4-vinylphenyl benzoate
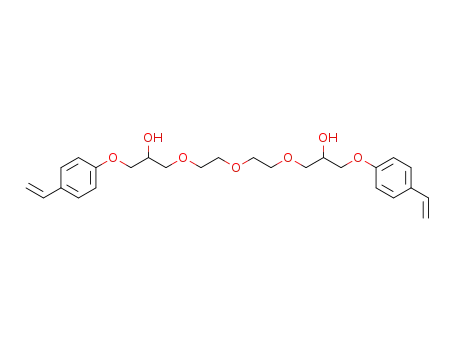
1-(2-{2-[2-Hydroxy-3-(4-vinyl-phenoxy)-propoxy]-ethoxy}-ethoxy)-3-(4-vinyl-phenoxy)-propan-2-ol
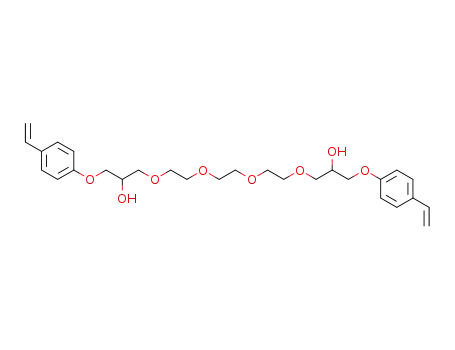
1-[2-(2-{2-[2-Hydroxy-3-(4-vinyl-phenoxy)-propoxy]-ethoxy}-ethoxy)-ethoxy]-3-(4-vinyl-phenoxy)-propan-2-ol
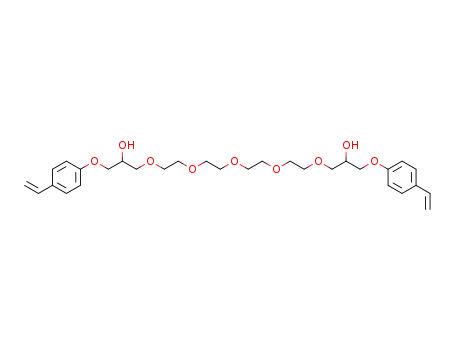
1-{2-[2-(2-{2-[2-Hydroxy-3-(4-vinyl-phenoxy)-propoxy]-ethoxy}-ethoxy)-ethoxy]-ethoxy}-3-(4-vinyl-phenoxy)-propan-2-ol
CAS:112163-33-4
CAS:112-34-5
CAS:24696-26-2
CAS:136572-09-3