- +86-0533-2185556
- WhatsApp: +86 15965530500
- admin@hangyubiotech.com
Your Location:Home >Products >small-molecule inhibitor >1192500-31-4
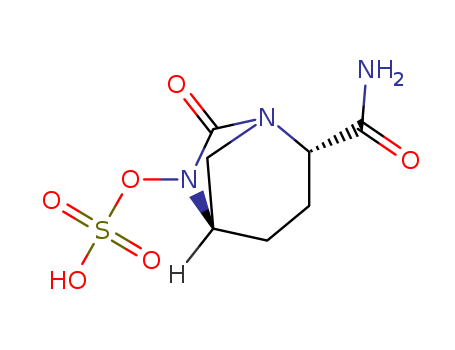

Purity:99%
The invention discloses a one-step metho...
An efficient synthesis of avibactam star...
The present invention relates to avibact...
The invention relates to a method for sy...
![(1R,2S,5R)-6-(benzyloxy)-7-oxo-1,6-diazabicyclo[3.2.1]octane-2-carboxamide](/upload/2025/4/697e6e99-a152-43d8-b4cc-3baf7c77c7fb.png)
(1R,2S,5R)-6-(benzyloxy)-7-oxo-1,6-diazabicyclo[3.2.1]octane-2-carboxamide

![(2S,5R)-2-carbamoyl-7-oxo-1,6-diazabicylco[3.2.1]octan-6-yl hydrogensulfate](/upload/2025/4/42000f21-2199-46b9-a67c-f9fab7cf49e0.png)
(2S,5R)-2-carbamoyl-7-oxo-1,6-diazabicylco[3.2.1]octan-6-yl hydrogensulfate
| Conditions | Yield |
|---|---|
|
(1R,2S,5R)-6-(benzyloxy)-7-oxo-1,6-diazabicyclo[3.2.1]octane-2-carboxamide;
With
hydrogen;
5% Pd-C;
In
dichloromethane; N,N-dimethyl-formamide;
under 2250.23 Torr;
With
sulfur trioxide-N,N-dimethylformamide complex; acetic acid;
In
dichloromethane; N,N-dimethyl-formamide;
|
|
|
With
palladium 10% on activated carbon; hydrogen; sulfur trioxide trimethylamine complex; triethylamine;
In
water; isopropyl alcohol;
for 5h;
Large scale;
Industrial scale;
|
|
|
With
palladium 10% on activated carbon; water; hydrogen; sulfur trioxide trimethylamine complex; triethylamine;
In
isopropyl alcohol;
|
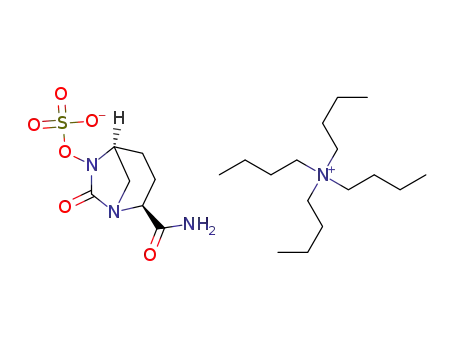
avibactam tetrabutylammonium salt

![(2S,5R)-2-carbamoyl-7-oxo-1,6-diazabicylco[3.2.1]octan-6-yl hydrogensulfate](/upload/2025/4/42000f21-2199-46b9-a67c-f9fab7cf49e0.png)
(2S,5R)-2-carbamoyl-7-oxo-1,6-diazabicylco[3.2.1]octan-6-yl hydrogensulfate
| Conditions | Yield |
|---|---|
|
With
toluene-4-sulfonic acid;
In
dichloromethane;
at 0 - 5 ℃;
for 0.5h;
Solvent;
Reagent/catalyst;
Temperature;
Concentration;
|
0.73 g |
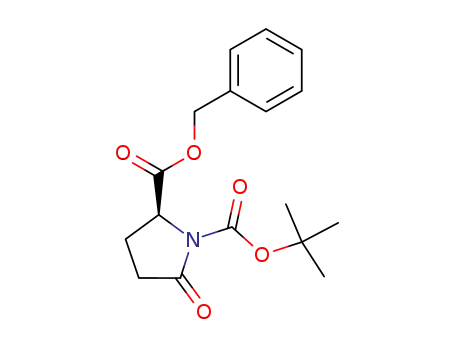
benzyl (2S)-N-tert-butoxycarbonyl-5-oxoprolinate
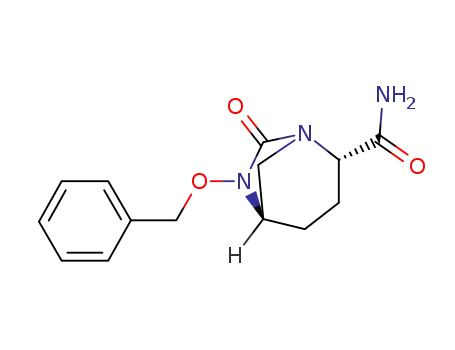
(1R,2S,5R)-6-(benzyloxy)-7-oxo-1,6-diazabicyclo[3.2.1]octane-2-carboxamide
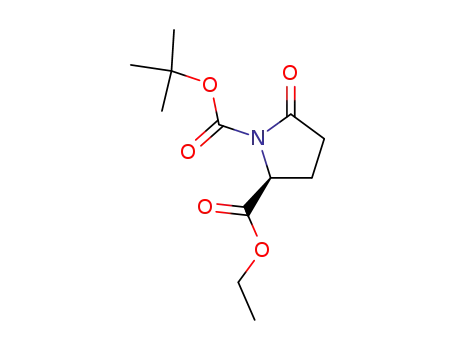
(S)-ethyl N-tert-butoxycarbonylpyroglutamate
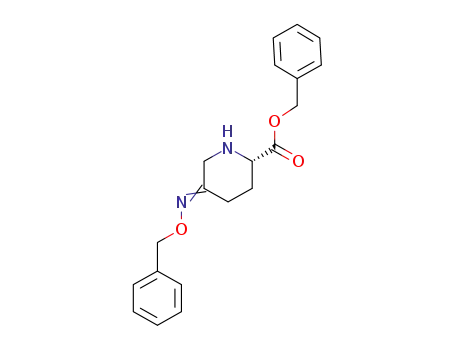
(2S)-5-(benzyloxyimino)-piperidine-2-carboxylic acid benzyl ester
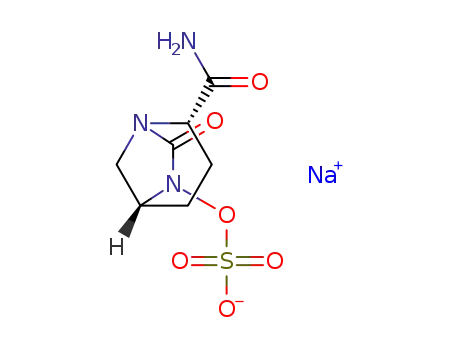
(2S,5R)-1,6-diazabicyclo[3.2.1]octane-2-carboxamide 7-oxo-6-(sulfoxy)monosodium salt
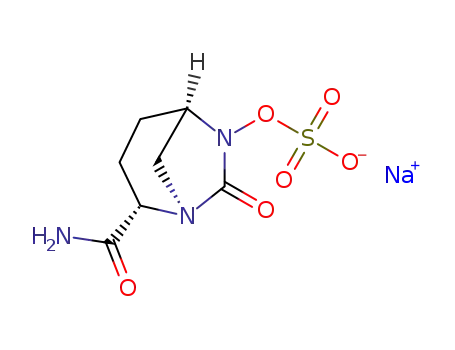
sulfuric acid [(1R,2S,5R)-2-aminocarbonyl-7-oxo-1,6-azabicyclo[3.2.1]oct-6-yl]ester sodium salt
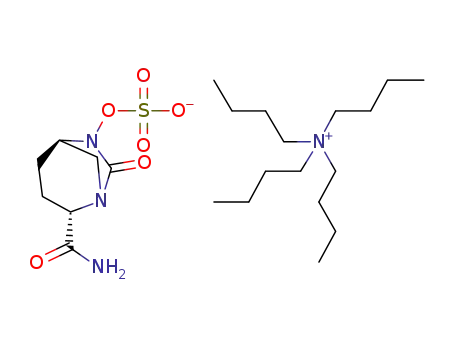
({[(2S,5R)-2-carbamoyl-7-oxo-6-(sulfooxy)-1,6-diazabicyclo[3,2,1]-oct-6-yl]oxy}sulfonyl)tetrabutylammonium salt
CAS:115473-15-9
CAS:1173-88-2
CAS:2649467-58-1