- +86-0533-2185556
- WhatsApp: +86 15965530500
- admin@hangyubiotech.com
Your Location:Home >Products >API >868540-17-4
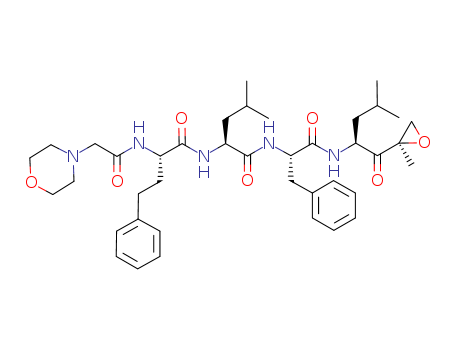

Purity:99%
|
Mechanism |
Carfilzomib is a tetrapeptide-based epoxy-proteasome inhibitor that irreversibly binds to the 20S proteasome containing the threonine N-terminal active site and the in vivo proteolysis core particle of 26S proteasome. In animal, carfilzomib has antiproliferative and apoptosis activity in vitro in solid and hematological granulocytes. In animals, carfilzomib inhibits proteasome activity in blood and tissue and delays tumor growth in multiple myeloma, hematological and solid tumor models. |
|
Side effects |
The most common adverse events observed in clinical trials (incidence ≥30%) were fatigue, anemia, nausea, thrombocytopenia, dyspnea, diarrhea and fever; the most common serious adverse events (overall incidence 45%) include pneumonia, acute renal failure, fever and congestive heart failure. |
|
Efficacy and Safety |
To assess the safety and efficacy of the drug, one study included 266 patients who had received at least two prior therapies, including bortezomib and thalidomide. It was assessed of the complete or partial disappearance of the tumor in the treated patient (overall response rate). The overall response rate was 23% and the median response time was 7.8 months. Common adverse effects of carfilzomib were observed in more than 30% of the subjects include fatigue, low blood cell counts and platelet counts, dyspnea, diarrhea and fever. Severe adverse reactions include heart failure and dyspnea. Upon the occurrence of serious adverse reactions, the patient should be closely monitored and stop the drug treatment. Information regarding the pharmacological effects, indications, mechanism of action, indications and side effects of Carfilzomib, a drug for treating multiple myeloma (MM), were compiled and edited by Xiao Nan of lookchem. |
|
Synthesis |
Carfilzomib is an analog of the natural product epoxomicin which was first synthesized in the laboratories of Professor Crews at Yale University. Subsequent development of the SAR led to the discovery of YU-101 in which 3 of the amino acids of this pentapeptide were modified to improve the potency of the molecule. After licensing the molecule to Proteolix, the introduction of the morpholino group was found to improve the solubility of the drug while maintaining efficient interaction with the target. The most scalable route to carfilzomib closely resembles the original route developed toward epoximicin and is described herein.The synthesis was initiated with the amide coupling of phenyl alanine methyl ester (53) and N-Boc leucine (54) using standard coupling reagents to afford dipeptide 55 in high yield the Scheme below. Acidic removal of the amine protecting group followed by a second amide coupling reaction with N-Boc homophenyl alanine provided tripeptide 56 in 85% yield for the two steps. Acidic removal of the amine protecting group followed by acylation with chloroacetyl chloride provided β-chloro amide 57 in 67% yield. Reaction of 57 with morpholine in the presence of catalytic amounts of potassium iodide followed by saponification of the methyl ester with lithium hydroxide provided acid 58 in 87% yield for the two steps. Amide coupling between acid 58 and keto-epoxyamine 59 (whose preparation is described in the scheme below) using HOBT as the coupling reagent followed by recrystallization of the resulting product ultimately gave carfilzomib (IX) in 75% yield. Keto-epoxyamine 59 was prepared from N-Boc leucine (54) as described in the Scheme below. Reaction of 54 with isobutyl chloroformate followed by N,O-dimethylhydroxylamine provided Weinreb amide 60 in 94% yield. Grignard addition of isopropenylmagnesium bromide 60 provided enone 62 in 81% yield. Epoxidation of 62 with calcium hypochlorite provided a mixture of epoxides giving 41% yield of the desired isomer (presumably isolated by chromatography), and subsequent treatment with TFA liberated the amine, providing the TFA salt of ketoepoxy amine 59 in 92% yield. |
|
Drug interactions |
Potentially hazardous interactions with other drugs Antipsychotics: avoid with clozapine - increased risk of agranulocytosis. |
|
Metabolism |
Carfilzomib was rapidly and extensively metabolised by mainly peptidase cleavage and epoxide hydrolysis. Cytochrome P450 mediated mechanisms played a minor role in overall carfilzomib metabolism. The metabolites have no known biologic activity. |
|
description |
Carfilzomib is an irreversible proteasome inhibitor and antineoplastic agent.The mechanism of action of carfilzomib is as a Proteasome Inhibitor, Carfilzomib irreversibly binds to and inhibits the chymotrypsin-like activity of the 20S catalytic core subunit of the proteasome, a protease complex responsible for degrading a large variety of cellular proteins. Inhibition of proteasome-mediated proteolysis results in an accumulation of polyubiquinated proteins, which may lead to cell cycle arrest, induction of apoptosis, and inhibition of tumor growth. Carfilzomib is used in treatment of refractory multiple myeloma. Carfilzomib is associated with a low rate of serum enzyme elevations during treatment and has been implicated to rare instances of clinically apparent, acute liver injury some of which have been fatal. Carfilzomib is a second-generation, irreversible, peptide epoxyketone class proteasome inhibitor that targets the chymotrypsin-like β5 subunit of the constitutive 20S proteasome (IC50 = 5.2 nM) and the β5i subunit of the immunoproteasome 20Si (LMP7; IC50 = 14 nM) with minimal cross reactivity to other proteases. It can induce cell cycle arrest and apoptosis in human cancer cell lines including multiple myeloma, lymphoma, and various solid tumors (IC50s = 2.4-20 nM). |
|
Definition |
ChEBI: A synthetic tetrapeptide consisting of morpholin-4-acetyl, L-2-amino-4-phenylbutanoyl, L-leucyl and L-phenylalanyl residues joined in sequence with the C-terminus connected to the amino group of (2 |
|
Brand name |
Kyprolis |
InChI:InChI=1/C40H57N5O7/c1-27(2)22-32(36(47)40(5)26-52-40)42-39(50)34(24-30-14-10-7-11-15-30)44-38(49)33(23-28(3)4)43-37(48)31(17-16-29-12-8-6-9-13-29)41-35(46)25-45-18-20-51-21-19-45/h6-15,27-28,31-34H,16-26H2,1-5H3,(H,41,46)(H,42,50)(H,43,48)(H,44,49)/t31-,32-,33-,34-,40+/m0/s1
Allenone has been identified as a highly...
A novel ynamide coupling reagent, the by...
The present invention relates to a proce...
The present invention relates to an impr...
![tert-butyl [(1S)-3-methyl-1-(2-methylpropyl)-2-oxobut-3-en-1-yl]carbamate](/upload/2025/4/abea0dce-da4f-4e64-83c8-25c898314759.png)
tert-butyl [(1S)-3-methyl-1-(2-methylpropyl)-2-oxobut-3-en-1-yl]carbamate

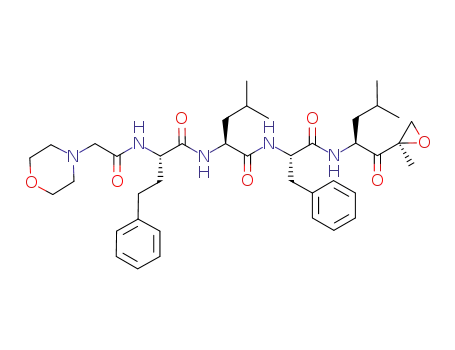
carfilzomib
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 3 steps
1: calcium hypochlorite / water; 1-methyl-pyrrolidin-2-one / 0.33 h / -15 - 0 °C
2: dichloromethane / 2 h / 0 - 20 °C / Inert atmosphere
3: benzotriazol-1-ol; N-ethyl-N,N-diisopropylamine; O-(1H-benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium hexafluorophosphate / N,N-dimethyl-formamide / 1.5 h / 0 - 5 °C
With calcium hypochlorite; benzotriazol-1-ol; O-(1H-benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium hexafluorophosphate; N-ethyl-N,N-diisopropylamine; In 1-methyl-pyrrolidin-2-one; dichloromethane; water; N,N-dimethyl-formamide;
|
|
|
Multi-step reaction with 3 steps
1: sodium hypochlorite; pyridine / water / 2 h / -5 - 0 °C
2: dichloromethane / 2 h / 0 - 20 °C / Inert atmosphere
3: benzotriazol-1-ol; N-ethyl-N,N-diisopropylamine; O-(1H-benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium hexafluorophosphate / N,N-dimethyl-formamide / 1.5 h / 0 - 5 °C
With pyridine; sodium hypochlorite; benzotriazol-1-ol; O-(1H-benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium hexafluorophosphate; N-ethyl-N,N-diisopropylamine; In dichloromethane; water; N,N-dimethyl-formamide;
|
|
|
Multi-step reaction with 3 steps
1: potassium carbonate; benzonitrile; dihydrogen peroxide / methanol / 6 h / 25 - 30 °C
2: trifluoroacetic acid / dichloromethane / 3 h / 5 - 10 °C / Inert atmosphere
3: dichloromethane / 1 h
With dihydrogen peroxide; potassium carbonate; benzonitrile; trifluoroacetic acid; In methanol; dichloromethane;
|
|
|
Multi-step reaction with 3 steps
1: potassium carbonate; benzonitrile; dihydrogen peroxide / methanol / 6 h / 25 - 30 °C
2: trifluoroacetic acid / dichloromethane / 3 h / 5 - 10 °C / Inert atmosphere
3: dichloromethane / 3 h / 25 - 30 °C
With dihydrogen peroxide; potassium carbonate; benzonitrile; trifluoroacetic acid; In methanol; dichloromethane;
|
|
|
Multi-step reaction with 5 steps
1: cerium(III) chloride heptahydrate; sodium tetrahydroborate / methanol / 1 h / 0 - 5 °C
2: 3-chloro-benzenecarboperoxoic acid / dichloromethane / 0 - 30 °C
3: Dess-Martin periodane / acetonitrile / 3 h / 0 - 30 °C
4: dichloromethane / 2 h / 2 - 30 °C
5: benzotriazol-1-ol; benzotriazol-1-yloxyl-tris-(pyrrolidino)-phosphonium hexafluorophosphate; N-ethyl-N,N-diisopropylamine / acetonitrile / 0 - 5 °C / Inert atmosphere
With sodium tetrahydroborate; cerium(III) chloride heptahydrate; benzotriazol-1-yloxyl-tris-(pyrrolidino)-phosphonium hexafluorophosphate; benzotriazol-1-ol; Dess-Martin periodane; N-ethyl-N,N-diisopropylamine; 3-chloro-benzenecarboperoxoic acid; In methanol; dichloromethane; acetonitrile;
|
|
|
Multi-step reaction with 6 steps
1.1: cerium(III) chloride heptahydrate; sodium tetrahydroborate / methanol / 1 h / 0 - 5 °C
2.1: 3-chloro-benzenecarboperoxoic acid / dichloromethane / 0 - 30 °C
3.1: Dess-Martin periodane / acetonitrile / 3 h / 0 - 30 °C
4.1: dichloromethane / 2 h / 2 - 30 °C
5.1: benzotriazol-1-ol; benzotriazol-1-yloxyl-tris-(pyrrolidino)-phosphonium hexafluorophosphate; N-ethyl-N,N-diisopropylamine / acetonitrile / -10 - -5 °C
5.2: 0.5 h / 25 - 30 °C
6.1: sodium hydrogencarbonate / dichloromethane; water / 0.17 h / 2 - 6 °C
With sodium tetrahydroborate; cerium(III) chloride heptahydrate; benzotriazol-1-yloxyl-tris-(pyrrolidino)-phosphonium hexafluorophosphate; sodium hydrogencarbonate; benzotriazol-1-ol; Dess-Martin periodane; N-ethyl-N,N-diisopropylamine; 3-chloro-benzenecarboperoxoic acid; In methanol; dichloromethane; water; acetonitrile;
|
|
|
Multi-step reaction with 8 steps
1.1: cerium(III) chloride heptahydrate; sodium tetrahydroborate / methanol / 1 h / 0 - 5 °C
2.1: 3-chloro-benzenecarboperoxoic acid / dichloromethane / 0 - 30 °C
3.1: Dess-Martin periodane / acetonitrile / 3 h / 0 - 30 °C
4.1: dichloromethane / 2 h / 2 - 30 °C
5.1: benzotriazol-1-ol; benzotriazol-1-yloxyl-tris-(pyrrolidino)-phosphonium hexafluorophosphate; N-ethyl-N,N-diisopropylamine / N,N-dimethyl-formamide / 2 - 6 °C
6.1: trifluoroacetic acid / dichloromethane / 2 - 6 °C
7.1: benzotriazol-1-ol; benzotriazol-1-yloxyl-tris-(pyrrolidino)-phosphonium hexafluorophosphate; N-ethyl-N,N-diisopropylamine / N,N-dimethyl-formamide / 2 - 6 °C
7.2: 0.5 h / 25 - 30 °C
8.1: sodium hydrogencarbonate / dichloromethane; water / 0.17 h / 2 - 6 °C
With sodium tetrahydroborate; cerium(III) chloride heptahydrate; benzotriazol-1-yloxyl-tris-(pyrrolidino)-phosphonium hexafluorophosphate; sodium hydrogencarbonate; benzotriazol-1-ol; Dess-Martin periodane; N-ethyl-N,N-diisopropylamine; 3-chloro-benzenecarboperoxoic acid; trifluoroacetic acid; In methanol; dichloromethane; water; N,N-dimethyl-formamide; acetonitrile;
|
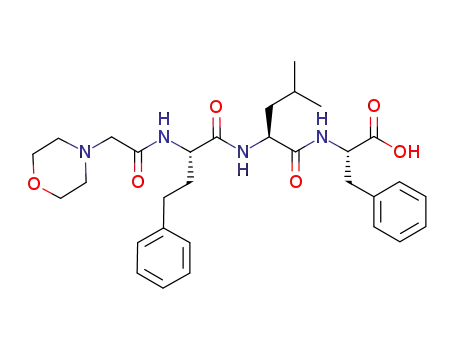
(S)-2-((S)-4-methyl-2-((S)-2-(2-morpholinoacetamido)-4-phenylbutanamido)pentanamido)-3-phenyipropanoic acid

C9H17NO2*C3HF5O2


carfilzomib
| Conditions | Yield |
|---|---|
|
With benzotriazol-1-ol; O-(1H-benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium hexafluorophosphate; diethylamine; In N,N-dimethyl-formamide; at -5 ℃; for 1.5h; Temperature; Reagent/catalyst;
|
86% |

(S)-2-((S)-4-methyl-2-((S)-2-(2-morpholinoacetamido)-4-phenylbutanamido)pentanamido)-3-phenyipropanoic acid
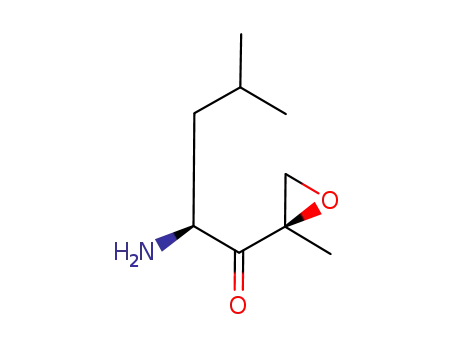
(2S)-2-amino-4-methyl-1-[(2R)-2-methyloxiran-2-yl]pentan-1-one
dichloromethane
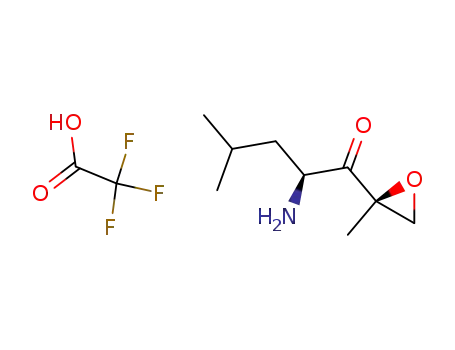
(S)-2-amino-4-methyl-1-((R)-2-methyloxiran-2-yl)pentan-1-one trifluoroacetic acid salt
CAS:112163-33-4
CAS:112-84-5
CAS:516-55-2