- +86-0533-2185556
- WhatsApp: +86 15965530500
- admin@hangyubiotech.com
Your Location:Home >Products >Pharmaceutical intermediate >10458-14-7
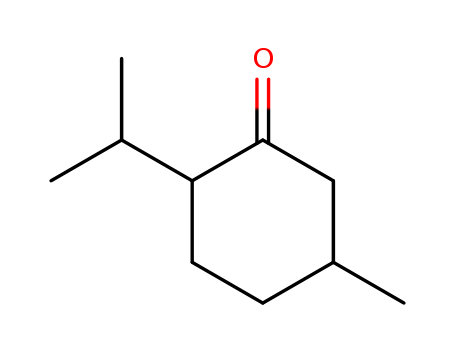

pd_meltingpoint:-6 °C
Appearance:Clear colourless liquid
Purity:99%
|
Chemical Description |
Menthone is a cyclic ketone with a minty odor. |
|
Synthesis Reference(s) |
The Journal of Organic Chemistry, 52, p. 5621, 1987 DOI: 10.1021/jo00234a021Tetrahedron Letters, 13, p. 749, 1972 |
|
General Description |
Menthone is a cyclic oxygenated terpene and its antioxidant potential has been evaluated. It is one of the key active component of Danshu capsule (DSC), a medicinal compound in traditional Chinese medicine. |
InChI:InChI=1/C10H18O/c1-7(2)9-5-4-8(3)6-10(9)11/h7-9H,4-6H2,1-3H3/t8-,9-/m0/s1
Oxidation of a wide variety of structura...
Herein we present a catalytic IBX-based ...
In an environmentally benign solventless...
The flavor menthon (isomeric mixture of ...
A simple and convenient method for the o...
NaBrO3 combined with NH4Cl is found to b...
Cu/SiO2 can be conveniently used for the...
Tetra-n-butylammonium per-ruthenate (Bun...
The realization of a synthetic biology a...
Catalytic amounts quaternary ammonium sa...
α,β-Unsaturated ketones which can readil...
A novel heterogenized Pd catalyst, Pd su...
This report discloses the photochemical ...
An excellent system for the selective ox...
Direct conversion of alkyl trimethylsily...
Conjugate addition of several compounds ...
A polymeric reagent electrochemically ge...
(Matrix presented) Oxidation of alcohols...
The title complex readily hydrogenates a...
A new system, I2-KI-K2CO3-H2O, selective...
Selective oxidation of secondary and ben...
Efficient aerobic oxidation of alcohols ...
Using a chiral (?)-menthone auxiliary, e...
Primary and secondary tetrahydropyranyl ...
The combination of (NH4)2Ce(NO3)6-NaBrO3...
-
Carbonyl compounds were regenerated from...
A variety of tetrahydropyranyl ethers ar...
The complex HOF·CH3CN, made directly fro...
Phosphonium-based Ionic Liquids (PhosILs...
HOF*CH3CN complex, made easily by bubbli...
The oxidation of secondary alcohols with...
-
Four NAD(P)H-dependent non-flavin ene re...
In the current work gold nanoparticles s...
An efficient protocol for the oxidation ...
The new trinuclear heteropolyperoxo comp...
A wide range of oxidative reactions are ...
Different types of alcohols are efficien...
Primary and secondary tetrahydropyranyl ...
-
The crystal structure of the title compo...
The NaBrO3/NaHSO3 reagent is one of the ...
The potential of cucurbit[5]uril to be u...
The efficiency of the bromide mediated b...
Hexamethylenetetramine-bromine supported...
The crystal structure of >*1.5H2O 1 is r...
The new mild oxidizing agent, quinoxalin...
Deprotection of O-allyl ether and in sit...
A novel selective fragmentation of cyclo...
The properties of high stability, period...
-
A visible-light-induced metal-free desul...
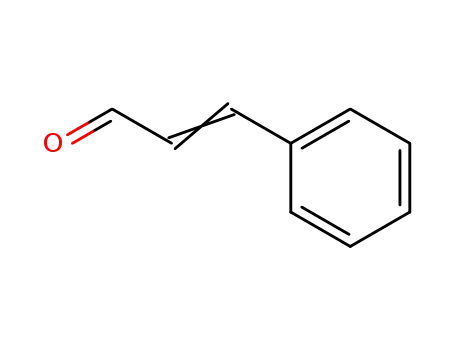
3-phenyl-propenal

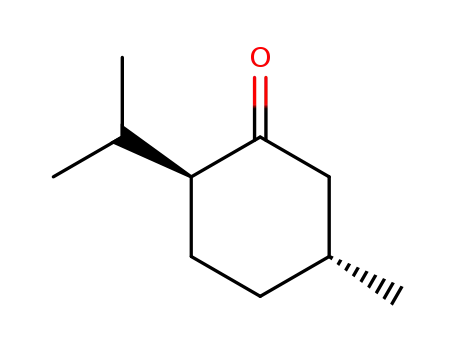
(2R,5S)-menthone

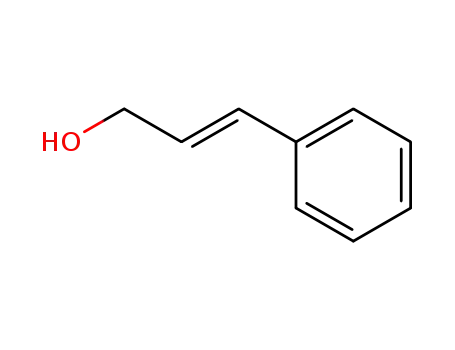
(2E)-3-phenyl-2-propen-1-ol
| Conditions | Yield |
|---|---|
|
With
(-)-menthol; aluminum isopropoxide;
under 5 Torr;
|
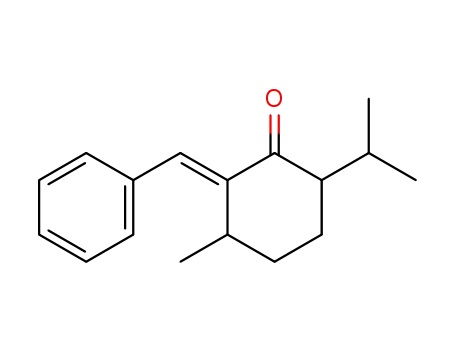
2-benzylidene-6-isopropyl-3-methyl-cyclohexanone

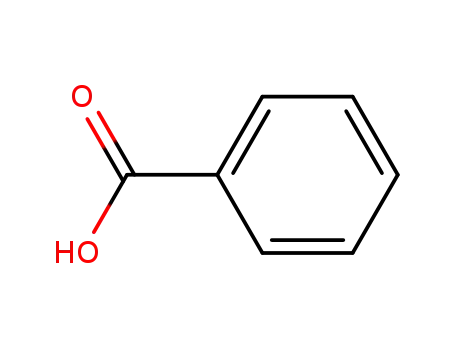
benzoic acid

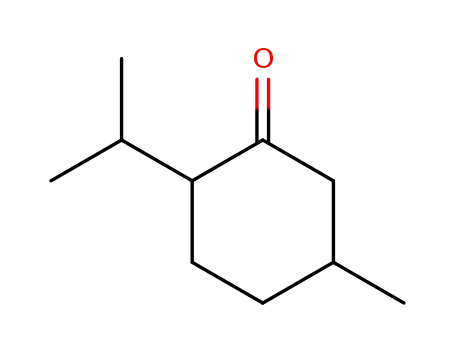
Menthone

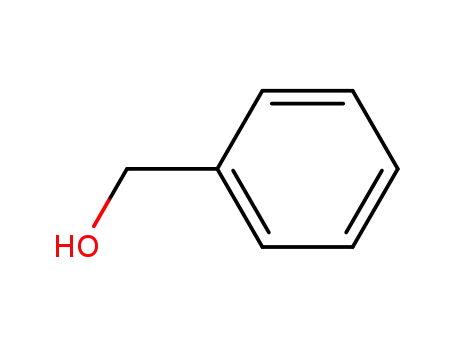
benzyl alcohol
| Conditions | Yield |
|---|---|
|
levorotatory substance;
|
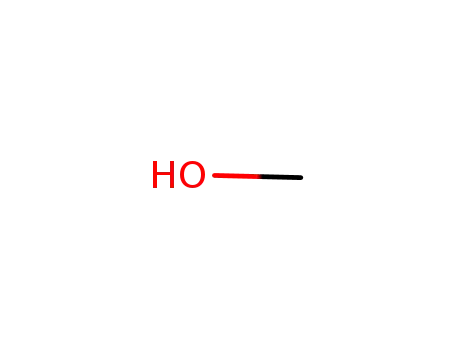
methanol
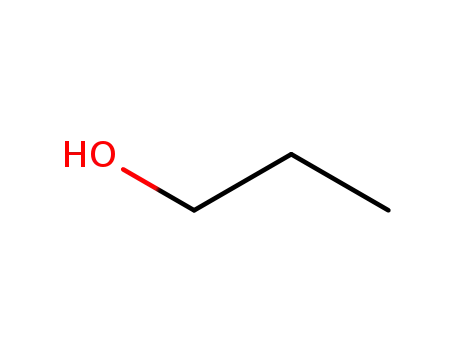
propan-1-ol
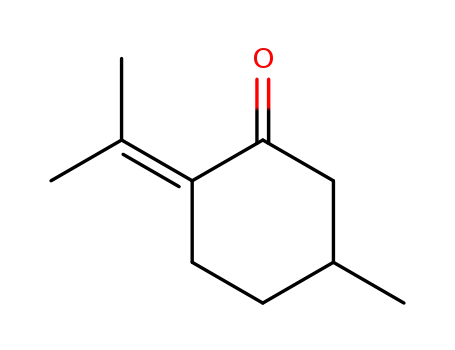
pulegone
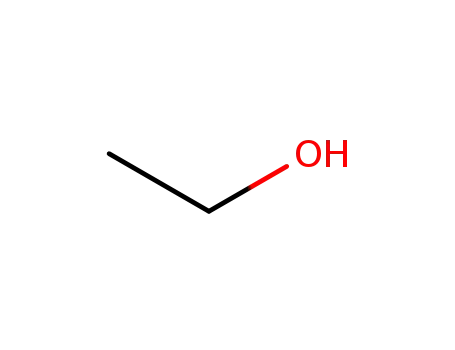
ethanol

2-benzylidene-6-isopropyl-3-methyl-cyclohexanone
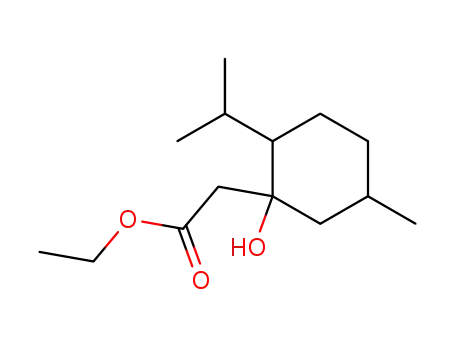
ethyl 2-isopropyl-5-methyl-1-hydroxycyclohexane-1-acetate
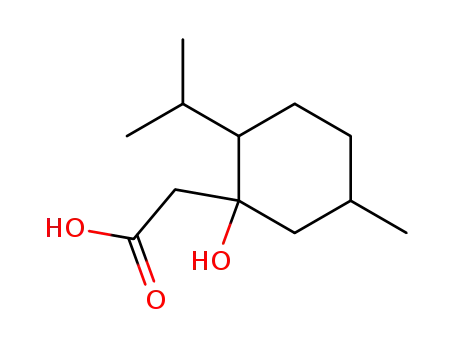
(3-hydroxy-p-menthan-3-yl)-acetic acid
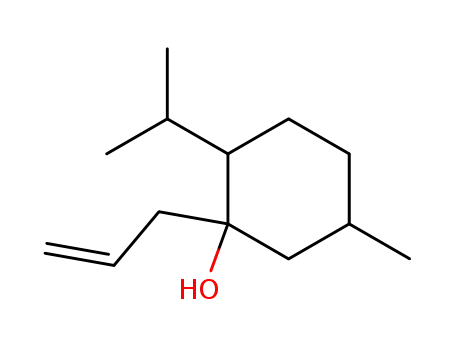
1-Allyl-2-isopropyl-5-methyl-cyclohexanol
CAS:112163-33-4
CAS:112-34-5
CAS:304896-28-4
CAS:675-09-2