- +86-0533-2185556
- WhatsApp: +86 15965530500
- admin@hangyubiotech.com

pd_meltingpoint:203-206 °C
Appearance:yellow crystal
Purity:99%
|
Biochem/physiol Actions |
Wogonin is an anti-inflammatory agent and COX-2 inhibitor, which inhibits the induction of both iNOS and COX-2. Wogonin inhibits COX-2 (IC50 = 46 μM) without affecting COX-1. Wogonin inhibits iNOS induction and thus inhibts activation-induced C6 glial cell death. Specifically, Wogonin inhibits NF-kappaB-mediated iNOS induction. |
|
Definition |
ChEBI: A dihydroxy- and monomethoxy-flavone in which the hydroxy groups are positioned at C-5 and C-7 and the methoxy group is at C-8. |
|
General Description |
Wogonin is a flavonoid component of Scutellaria baicalensis Georgi roots. |
InChI:InChI=1/C16H12O5/c1-20-15-12(19)7-10(17)14-11(18)8-13(21-16(14)15)9-5-3-2-4-6-9/h2-8,17,19H,1H3
A scalable and practical route to wogoni...
Wogonin and astringin were synthesized f...
The invention discloses a preparation me...
The invention discloses a preparation me...
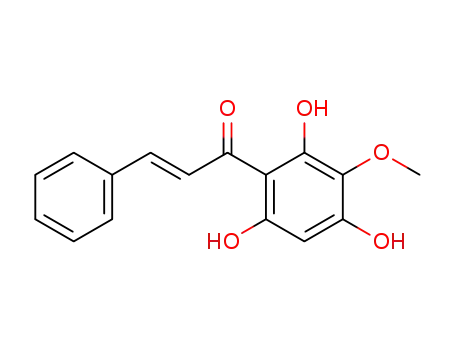
(E)-3-Phenyl-1-(2,4,6-trihydroxy-3-methoxy-phenyl)-propenone

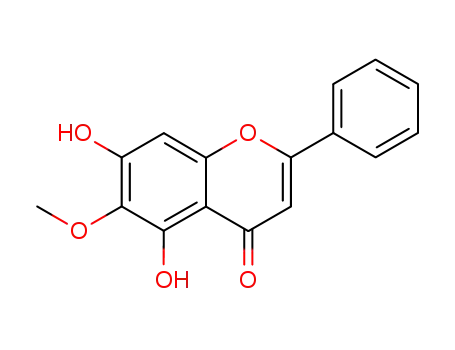
oroxylin A

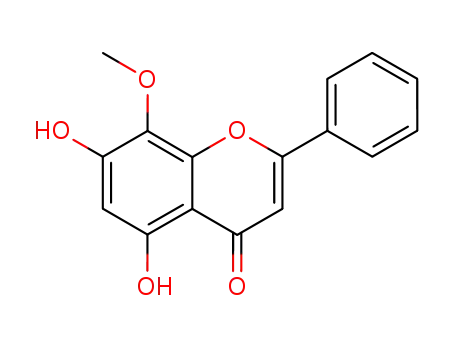
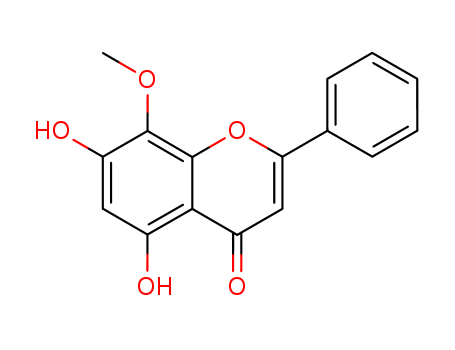
wogonin
| Conditions | Yield |
|---|---|
|
With iodine; dimethyl sulfoxide; Heating;
|
46% 24% |


oroxylin A


wogonin
| Conditions | Yield |
|---|---|
|
|
24% 46% |
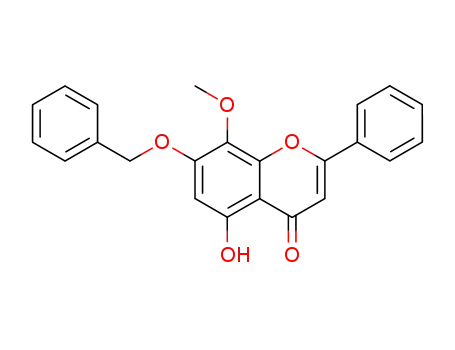
7-benzyloxy-5-hydroxy-8-methoxy-2-phenyl-4H-chromen-4-one

(E)-3-Phenyl-1-(2,4,6-trihydroxy-3-methoxy-phenyl)-propenone
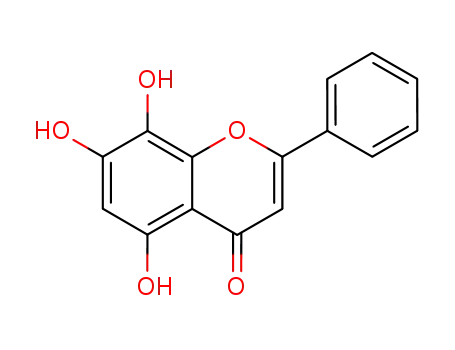
norwogonin
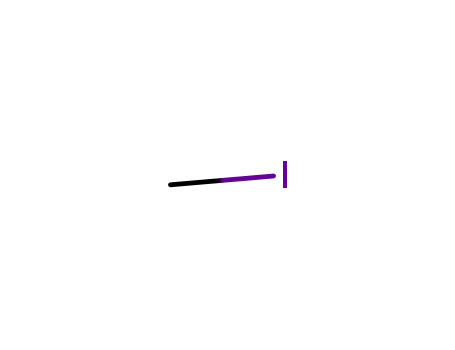
methyl iodide
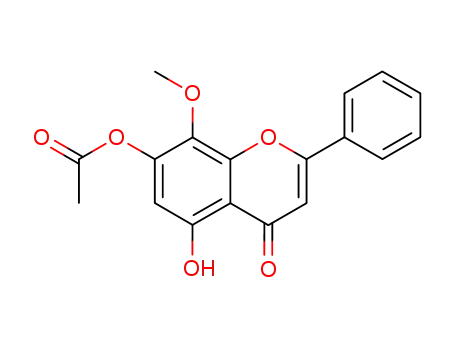
5-hydroxy-8-methoxy-4-oxo-2-phenyl-4H-chromen-7-yl acetate
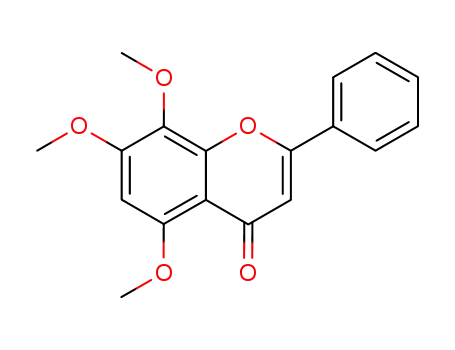
5,7,8-trimethoxy-2-phenyl-chromen-4-one

norwogonin
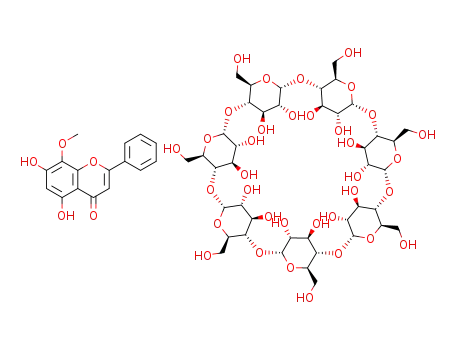
C16H12O5*C42H70O35
CAS:112163-33-4
CAS:112-34-5
CAS:42743-15-7
CAS:133256-15-2