- +86-0533-2185556
- WhatsApp: +86 15965530500
- admin@hangyubiotech.com
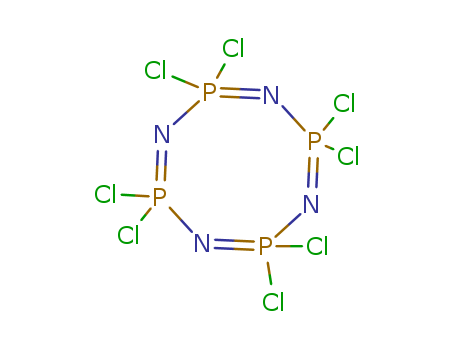

pd_meltingpoint:123-124 °C
Purity:99%
|
General Description |
2,2,4,4,6,6,8,8-octachloro-2,2,4,4,6,6,8,8-octahydro-1,3,5,7,2,4,6,8-tetraazatetraphosphocine is a compound with a highly complex and specific chemical structure. It contains eight chlorine atoms and eight hydrogen atoms attached to a central core made up of four nitrogen and four phosphorus atoms. 2,2,4,4,6,6,8,8-octachloro-2,2,4,4,6,6,8,8-octahydro-1,3,5,7,2,4,6,8-tetraazatetraphosphocine is highly toxic and is not naturally occurring in the environment. Its chemical structure makes it extremely stable and difficult to break down, presenting significant environmental and health risks if released into the environment. Due to its toxic nature, it is not commonly used and is strictly controlled and regulated. |
InChI:InChI=1/Cl8H8N4P4/c1-13(2)9-14(3,4)11-16(7,8)12-15(5,6)10-13/h9-16H
An object of the present invention is to...
The invention discloses octa-(1,2-dyhydr...
Medium-sized cyclic oligomeric phosphaze...
New insight into the mechanism of the am...
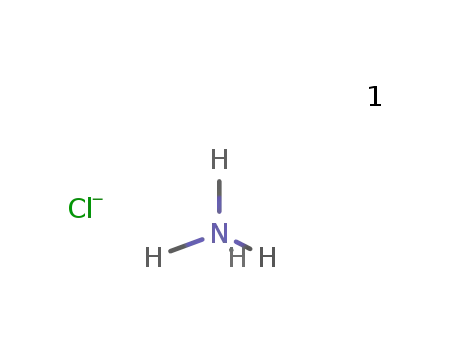
ammonium chloride

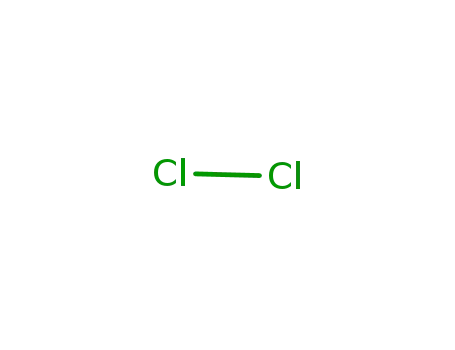
chlorine

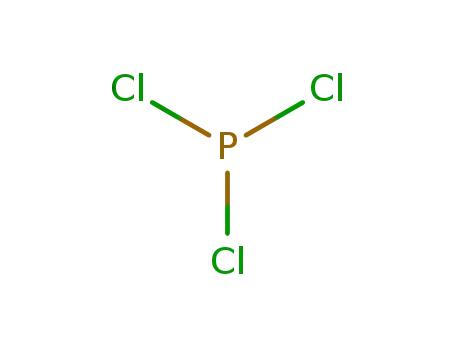
phosphorus trichloride

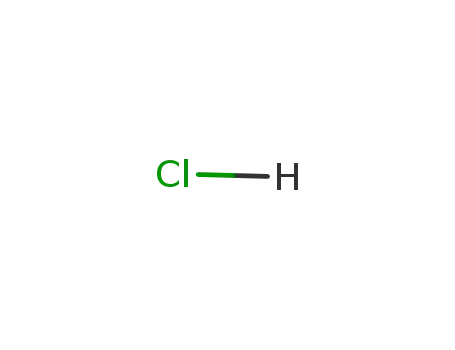
hydrogenchloride

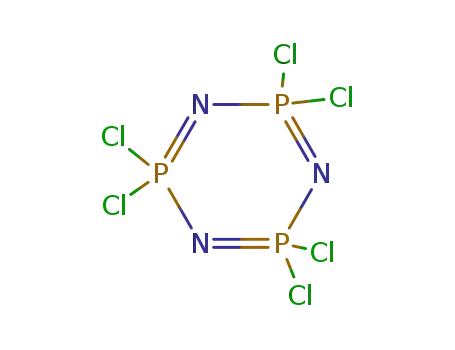
2,2,4,4,6,6-hexachloro-1,3,5-triaza-2,4,6-triphosphorine

cyclic polyphosphorus nitride dichloride

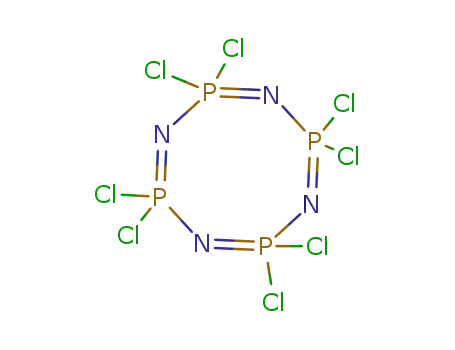
octachlorocyclotetraphosphazene
| Conditions | Yield |
|---|---|
|
In further solvent(s); dissolving PCl3 distd. under N2 in (CHCl2)2 distd. under N2; addn. of NH4Cl dryed in vac. and passing in the corresponding amount of Cl2; heating in a stream of N2 at 130 °C; removal of formed HCl by a stream of N2;; filtration, distg. off the solvent at 40 °C and 0.5 Torr; recrystn. from petroleum ether; products (NPCl2)3, (NPCl2)4 and a small amount of oily polymers;;
|
|
|
In chlorobenzene; dissolving PCl3 distd. under N2 in C6H5Cl distd. under N2; addn. of NH4Cl dryed in vac. and passing in the corresponding amount of Cl2; heating in a stream of N2 at 130 °C; removal of formed HCl by a stream of N2;; filtration, distg. off the solvent at 40 °C and 0.5 Torr; recrystn. from petroleum ether; product contains small amounts of higher polymers;;
|
|
|
In further solvent(s); dissolving PCl3 distd. under N2 in (CHCl2)2 distd. under N2; addn. of NH4Cl dryed in vac. and passing in the corresponding amount of Cl2; heating in a stream of N2 at 130 °C; removal of formed HCl by a stream of N2;; filtration, distg. off the solvent at 40 °C and 0.5 Torr; recrystn. from petroleum ether; yield 35-40 %, product contains small amounts of higher polymers;;
|
35-40 |
|
|
|
|
In chlorobenzene;
|
P3N7H12


phosphorus trichloride


hydrogenchloride


octachlorocyclotetraphosphazene
| Conditions | Yield |
|---|---|
|
In not given; reaction with an equimolar amount of PCl3 under formation of P4N4Cl8 and evolution of 2 mol HCl;;
|
|
|
In not given; reaction with an equimolar amount of PCl3 under formation of P4N4Cl8 and evolution of 2 mol HCl;;
|
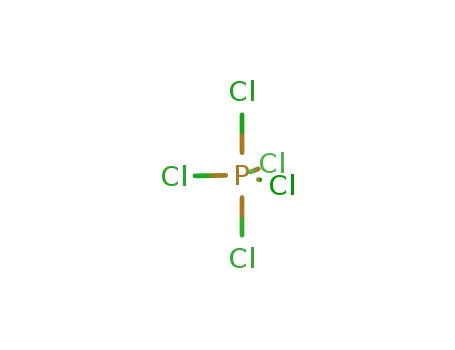
phosphorus pentachloride
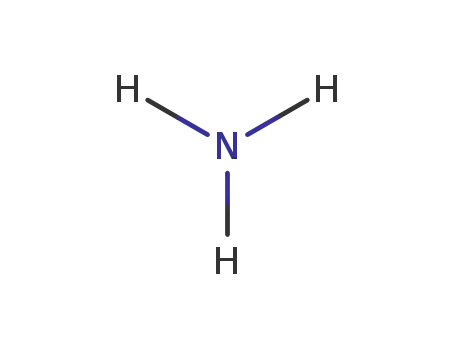
ammonia

chlorine

phosphorus trichloride

hydrogenchloride
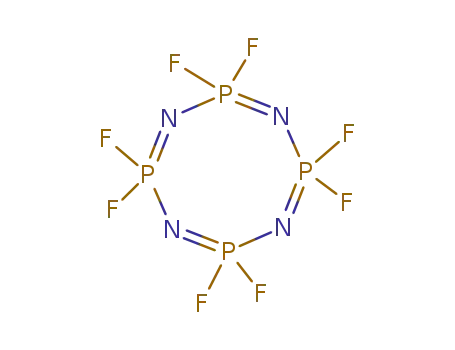
octafluorophosphazene
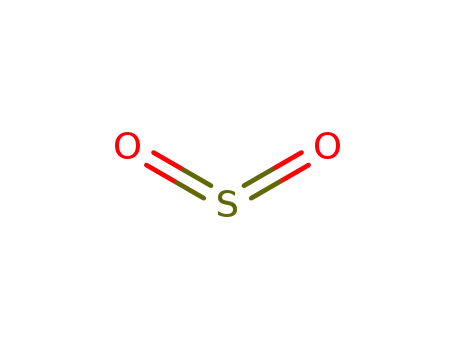
sulfur dioxide
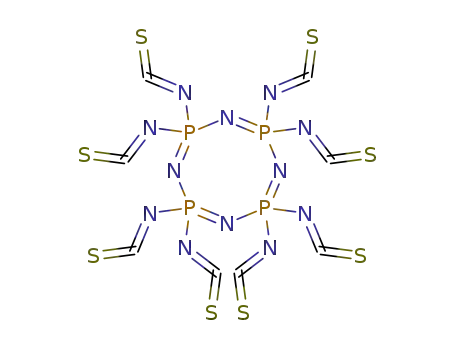
2,2,4,4,6,6,8,8-octaisothiocyanato-1,3,5,7-tetraza-2λ5,4λ5,6λ5,8λ5-tetraphosphacycloocta-1,3,5,7-tetraene
CAS:115473-15-9
CAS:118685-33-9
CAS:133256-15-2
CAS:2157-01-9