- +86-0533-2185556
- +86 15965530500
- admin@hangyubiotech.com
Your Location:Home >Products >small-molecule inhibitor >1883727-34-1

Purity:99%
Provided herein are myeloid cell leukemi...
Provided herein are myeloid cell leukemi...
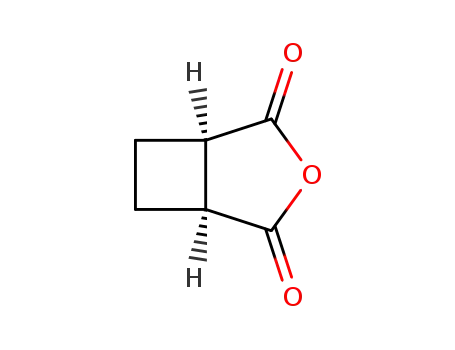
cis-1,2-cyclobutane dicarboxylic anhydride

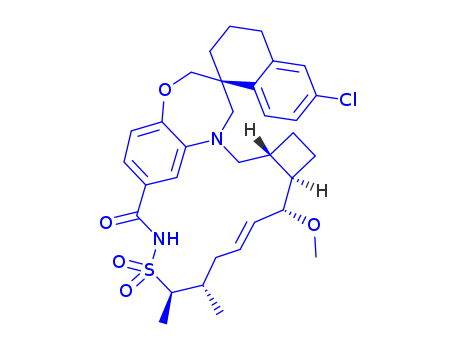
![(1S,3’R,6’R,7’S,9’E,11’S,12’R)-6-chloro-7'-methoxy-11’,12’-dimethyl-3,4-dihydro-2H,15’H-spiro[naphthalene-1,22’-[20]oxa[13]thia[1,14]diazatetracyclo[14.7.2.03,6.019,24]pentacosa[9,16,18,24]tetraen]-15’-one-13’,13'-dioxide](/upload/2025/4/ee4f7a38-467d-431b-b685-d339dea71da4.png)
(1S,3’R,6’R,7’S,9’E,11’S,12’R)-6-chloro-7'-methoxy-11’,12’-dimethyl-3,4-dihydro-2H,15’H-spiro[naphthalene-1,22’-[20]oxa[13]thia[1,14]diazatetracyclo[14.7.2.03,6.019,24]pentacosa[9,16,18,24]tetraen]-15’-one-13’,13'-dioxide
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 12 steps
1.1: lithium aluminium tetrahydride / tetrahydrofuran / 18 h / 20 - 50 °C / Inert atmosphere
2.1: 50 °C
3.1: sodium citrate / water / 0.2 °C / pH Ca545 / Enzymatic reaction
4.1: 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical; [bis(acetoxy)iodo]benzene / dichloromethane / 15 - 20 °C
4.2: 48 h / 1.8 - 20 °C
5.1: dichloromethane; acetic acid / 0.17 h / 20 °C
5.2: 1.17 h / 0 °C
6.1: potassium hydroxide / methanol / 4 h / 20 °C
6.2: pH 7
7.1: dimethyl sulfoxide; oxalyl dichloride; triethylamine / dichloromethane / 2.5 h / -70 °C / Inert atmosphere
8.1: (1R,2S)-2-(morpholin-4-yl)-1-phenyl-1-propanol; n-butyllithium / toluene; hexane / 1 h / 0 - 12 °C
8.2: 0 h / -10 - -5 °C
9.1: water; lithium hydroxide monohydrate / tetrahydrofuran; methanol / 4 h / 50 °C
9.2: 0 °C / pH 3
10.1: 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; dmap / dichloromethane / 0 - 20 °C
11.1: Hoveyda-Grubbs catalyst second generation / dichloromethane / 15 h / 45 °C / Inert atmosphere
12.1: potassium hexamethylsilazane / tetrahydrofuran; 2-methyltetrahydrofuran / 2 h / -44 - 20 °C / Inert atmosphere
With
dmap; (1R,2S)-2-(morpholin-4-yl)-1-phenyl-1-propanol; lithium aluminium tetrahydride; n-butyllithium; Hoveyda-Grubbs catalyst second generation; oxalyl dichloride; 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical; lithium hydroxide monohydrate; [bis(acetoxy)iodo]benzene; water; sodium citrate; potassium hexamethylsilazane; dimethyl sulfoxide; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; triethylamine; potassium hydroxide;
In
tetrahydrofuran; 2-methyltetrahydrofuran; methanol; hexane; dichloromethane; water; acetic acid; toluene;
|
|
|
Multi-step reaction with 12 steps
1.1: lithium aluminium tetrahydride / tetrahydrofuran / 18 h / 20 - 50 °C / Inert atmosphere
2.1: 50 °C
3.1: sodium citrate / water / 0.2 °C / pH Ca545 / Enzymatic reaction
4.1: 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical; [bis(acetoxy)iodo]benzene / dichloromethane / 15 - 20 °C
4.2: 48 h / 1.8 - 20 °C
5.1: dichloromethane; acetic acid / 0.17 h / 20 °C
5.2: 1.17 h / 0 °C
6.1: potassium hydroxide / methanol / 4 h / 20 °C
6.2: pH 7
7.1: dimethyl sulfoxide; oxalyl dichloride; triethylamine / dichloromethane / 2.5 h / -70 °C / Inert atmosphere
8.1: (1R,2S)-2-(morpholin-4-yl)-1-phenyl-1-propanol; n-butyllithium / toluene; hexane / 1 h / 0 - 12 °C
8.2: 0 h / -10 - -5 °C
9.1: water; lithium hydroxide monohydrate / tetrahydrofuran; methanol / 4 h / 50 °C
9.2: 0 °C / pH 3
10.1: 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; dmap / dichloromethane / 0 - 20 °C
11.1: Hoveyda-Grubbs catalyst second generation / 1,2-dichloro-ethane / 10 h / 60 °C / Inert atmosphere
11.2: 20 °C
12.1: potassium hexamethylsilazane / tetrahydrofuran; 2-methyltetrahydrofuran / 2 h / -44 - 20 °C / Inert atmosphere
With
dmap; (1R,2S)-2-(morpholin-4-yl)-1-phenyl-1-propanol; lithium aluminium tetrahydride; n-butyllithium; Hoveyda-Grubbs catalyst second generation; oxalyl dichloride; 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical; lithium hydroxide monohydrate; [bis(acetoxy)iodo]benzene; water; sodium citrate; potassium hexamethylsilazane; dimethyl sulfoxide; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; triethylamine; potassium hydroxide;
In
tetrahydrofuran; 2-methyltetrahydrofuran; methanol; hexane; dichloromethane; water; acetic acid; 1,2-dichloro-ethane; toluene;
|

cis-1,2-cyclobutane dicarboxylic anhydride

(1S,3'R,6'R,7'S,8'Z,11'S,12'R)-6-chloro-7'-methoxy-11',12'-dimethyl-3,4-dihydro-2H,15'H-spiro[naphthalene-1,22'-[20]oxa[13]thia[1,14]diazatetracyclo[14.7.2.03'6.019'24]pentacosa[8,16,18,24]tetraen]-15'-one 13',13'-dioxide
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 12 steps
1.1: lithium aluminium tetrahydride / tetrahydrofuran / 18 h / 20 - 50 °C / Inert atmosphere
2.1: 50 °C
3.1: sodium citrate / water / 0.2 °C / pH Ca545 / Enzymatic reaction
4.1: 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical; [bis(acetoxy)iodo]benzene / dichloromethane / 15 - 20 °C
4.2: 48 h / 1.8 - 20 °C
5.1: dichloromethane; acetic acid / 0.17 h / 20 °C
5.2: 1.17 h / 0 °C
6.1: potassium hydroxide / methanol / 4 h / 20 °C
6.2: pH 7
7.1: dimethyl sulfoxide; oxalyl dichloride; triethylamine / dichloromethane / 2.5 h / -70 °C / Inert atmosphere
8.1: (1R,2S)-2-(morpholin-4-yl)-1-phenyl-1-propanol; n-butyllithium / toluene; hexane / 1 h / 0 - 12 °C
8.2: 0 h / -10 - -5 °C
9.1: water; lithium hydroxide monohydrate / tetrahydrofuran; methanol / 4 h / 50 °C
9.2: 0 °C / pH 3
10.1: 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; dmap / dichloromethane / 0 - 20 °C
11.1: Hoveyda-Grubbs catalyst second generation / dichloromethane / 15 h / 45 °C / Inert atmosphere
12.1: sodium hydride / tetrahydrofuran; mineral oil / 0.33 h / 0 °C
12.2: 1 h / 20 °C
With
dmap; (1R,2S)-2-(morpholin-4-yl)-1-phenyl-1-propanol; lithium aluminium tetrahydride; n-butyllithium; Hoveyda-Grubbs catalyst second generation; oxalyl dichloride; 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical; lithium hydroxide monohydrate; [bis(acetoxy)iodo]benzene; water; sodium citrate; sodium hydride; dimethyl sulfoxide; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; triethylamine; potassium hydroxide;
In
tetrahydrofuran; methanol; hexane; dichloromethane; water; acetic acid; toluene; mineral oil;
|
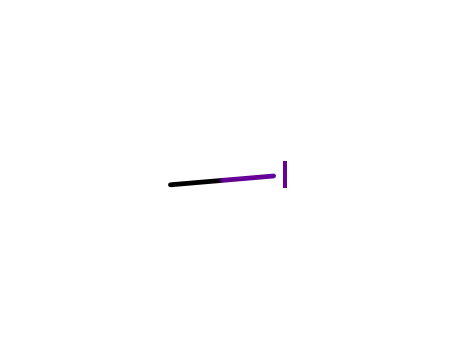
methyl iodide
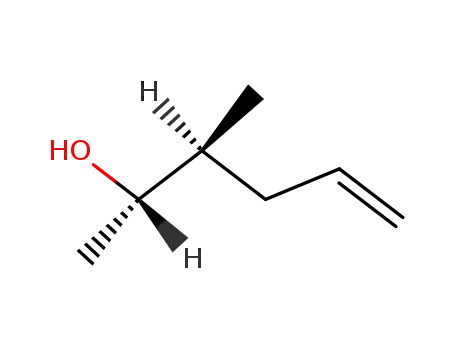
(2S,3S)-3-Methyl-5-hexen-2-ol
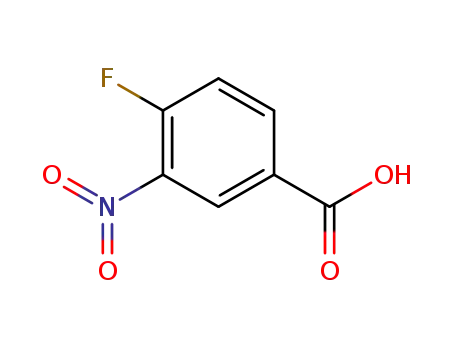
3-nitro-4-fluorobenzoic acid
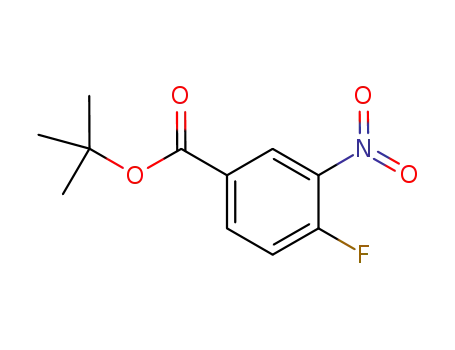
tert-butyl 4-fluoro-3-nitrobenzoate
CAS:936563-96-1
CAS:10039-32-4
CAS:2157-01-9
CAS:187820-08-2