- +86-0533-2185556
- WhatsApp: +86 15965530500
- admin@hangyubiotech.com
Your Location:Home >Products >API >936563-96-1

Purity:99%
The present invention provides a prepara...
The invention relates to a preparation m...
The invention relates to a preparation m...
The invention discloses a purification m...
![2-[(4-phenoxy-phenyl)-methoxy-methylene]-malononitrile](/upload/2025/4/72777294-39e3-4068-b056-661481141525.png)
2-[(4-phenoxy-phenyl)-methoxy-methylene]-malononitrile

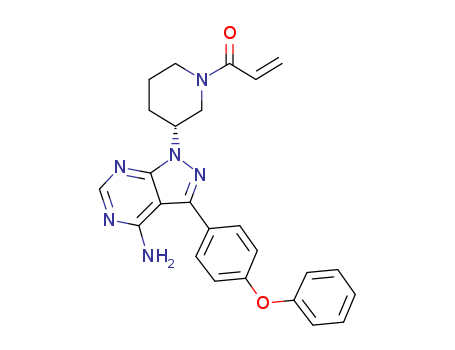
ibrutinib
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 4 steps
1.1: hydrazine hydrate / methanol / 2 h / 25 - 30 °C
2.1: zinc(II) chloride / o-xylene / 20.17 h / 25 - 120 °C
3.1: triphenylphosphine / tetrahydrofuran / 0.17 h / 25 - 30 °C
3.2: 3 h / 45 - 60 °C
4.1: sodium carbonate / dichloromethane; water / 10 - 15 °C
4.2: 0.33 h / -45 - 30 °C
With sodium carbonate; hydrazine hydrate; triphenylphosphine; zinc(II) chloride; In tetrahydrofuran; methanol; dichloromethane; o-xylene; water;
|
|
|
Multi-step reaction with 5 steps
1.1: hydrazine hydrate / methanol / 2 h / 25 - 30 °C
2.1: zinc(II) chloride / o-xylene / 20.17 h / 25 - 120 °C
3.1: caesium carbonate / 1-methyl-pyrrolidin-2-one / 12 h / 70 - 75 °C / Inert atmosphere
4.1: hydrogenchloride / ethyl acetate; isopropyl alcohol / 16 h / 25 - 30 °C
5.1: sodium carbonate / dichloromethane; water / 10 - 15 °C
5.2: 0.33 h / -45 - 30 °C
With hydrogenchloride; sodium carbonate; caesium carbonate; hydrazine hydrate; zinc(II) chloride; In 1-methyl-pyrrolidin-2-one; methanol; dichloromethane; o-xylene; water; ethyl acetate; isopropyl alcohol;
|
|
|
Multi-step reaction with 5 steps
1.1: hydrazine hydrate / methanol / 2 h / 25 - 30 °C
2.1: zinc(II) chloride / o-xylene / 20.17 h / 25 - 120 °C
3.1: triphenylphosphine / tetrahydrofuran / 0.17 h / 25 - 30 °C
3.2: 3 h / 45 - 60 °C
4.1: sodium carbonate / dichloromethane; water / 10 - 15 °C
4.2: 0.33 h / -45 - 30 °C
5.1: triethylamine; hydroquinone; 1,8-diazabicyclo[5.4.0]undec-7-ene / acetonitrile / 16 h / 25 - 30 °C
With sodium carbonate; hydrazine hydrate; 1,8-diazabicyclo[5.4.0]undec-7-ene; triethylamine; hydroquinone; triphenylphosphine; zinc(II) chloride; In tetrahydrofuran; methanol; dichloromethane; o-xylene; water; acetonitrile;
|
|
|
Multi-step reaction with 6 steps
1.1: hydrazine hydrate / methanol / 2 h / 25 - 30 °C
2.1: zinc(II) chloride / o-xylene / 20.17 h / 25 - 120 °C
3.1: caesium carbonate / 1-methyl-pyrrolidin-2-one / 12 h / 70 - 75 °C / Inert atmosphere
4.1: hydrogenchloride / ethyl acetate; isopropyl alcohol / 16 h / 25 - 30 °C
5.1: sodium carbonate / dichloromethane; water / 10 - 15 °C
5.2: 0.33 h / -45 - 30 °C
6.1: triethylamine; hydroquinone; 1,8-diazabicyclo[5.4.0]undec-7-ene / acetonitrile / 16 h / 25 - 30 °C
With hydrogenchloride; sodium carbonate; caesium carbonate; hydrazine hydrate; 1,8-diazabicyclo[5.4.0]undec-7-ene; triethylamine; hydroquinone; zinc(II) chloride; In 1-methyl-pyrrolidin-2-one; methanol; dichloromethane; o-xylene; water; ethyl acetate; isopropyl alcohol; acetonitrile;
|
|
|
Multi-step reaction with 5 steps
1.1: hydrazine hydrate / ethanol / 1 h / Reflux
2.1: 8 h / 150 °C
3.1: potassium carbonate / N,N-dimethyl-formamide / 2 h / 21 - 22 °C
3.2: 14 h / 80 °C
4.1: hydrogenchloride / ethyl acetate / 0.5 h
5.1: triethylamine / dichloromethane
With hydrogenchloride; potassium carbonate; hydrazine hydrate; triethylamine; In ethanol; dichloromethane; ethyl acetate; N,N-dimethyl-formamide;
|
|
|
Multi-step reaction with 5 steps
1.1: hydrazine hydrate / ethanol / 1 h / Reflux
2.1: 8 h / 150 °C
3.1: potassium carbonate / N,N-dimethyl-formamide / 2 h / 21 - 22 °C
3.2: 14 h / 80 °C
4.1: hydrogenchloride / ethyl acetate / 21 - 22 °C
5.1: triethylamine / methanol; tert-butyl methyl ether / -12 - -10 °C
With hydrogenchloride; potassium carbonate; hydrazine hydrate; triethylamine; In methanol; ethanol; tert-butyl methyl ether; ethyl acetate; N,N-dimethyl-formamide;
|
|
|
Multi-step reaction with 3 steps
1.1: triethylamine / ethanol / 1 h / 20 °C / Sonication
2.1: di-tert-butyl dicarbonate; triethylamine / dichloromethane / 2 h / 20 °C / Inert atmosphere
2.2: 2.5 h / 180 °C
3.1: sodium hydroxide / methanol / 7 h / Inert atmosphere; Reflux
3.2: 1 h / 20 °C
With di-tert-butyl dicarbonate; triethylamine; sodium hydroxide; In methanol; ethanol; dichloromethane;
|
|
|
Multi-step reaction with 5 steps
1.1: hydrazine hydrate / toluene / 25 - 50 °C
2.1: 135 - 140 °C
3.1: di-isopropyl azodicarboxylate; triphenylphosphine / tetrahydrofuran
3.2: 20 °C
4.1: trifluoroacetic acid / dichloromethane
5.1: triethylamine / tetrahydrofuran / 18 h / 25 - 30 °C
With di-isopropyl azodicarboxylate; hydrazine hydrate; triethylamine; triphenylphosphine; trifluoroacetic acid; In tetrahydrofuran; dichloromethane; toluene; 3.1: |Mitsunobu Displacement / 3.2: |Mitsunobu Displacement;
|
|
|
Multi-step reaction with 3 steps
1.1: triethylamine / ethanol / 1 h / 20 °C / Sonication; Green chemistry
2.1: 0.5 h / 100 °C / Inert atmosphere; Microwave irradiation; Green chemistry
3.1: sodium hydroxide; methanol / 7 h / Reflux; Green chemistry
3.2: 2 h / 20 °C / Green chemistry
With methanol; triethylamine; sodium hydroxide; In ethanol;
|
|
|
Multi-step reaction with 5 steps
1.1: ethanol / 0.5 h / 5 °C
1.2: 15 h / 5 - 25 °C
2.1: butan-1-ol / 19 h / 120 °C
3.1: hydrogen / water; methanol / 2 h / 50 °C / 1034.32 Torr / Acidic conditions
4.1: sodium hydrogencarbonate / water; 2-methyltetrahydrofuran
5.1: 1,8-diazabicyclo[5.4.0]undec-7-ene / ethyl acetate
With hydrogen; sodium hydrogencarbonate; 1,8-diazabicyclo[5.4.0]undec-7-ene; In 2-methyltetrahydrofuran; methanol; ethanol; water; ethyl acetate; butan-1-ol;
|
![2-[(4-phenoxy-phenyl)-methoxy-methylene]-malononitrile](/upload/2025/4/72777294-39e3-4068-b056-661481141525.png)
2-[(4-phenoxy-phenyl)-methoxy-methylene]-malononitrile

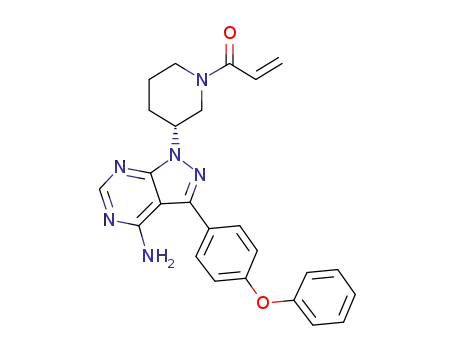
![1-[(3R)-3-[4-amino-3-(4-phenoxyphenyl)pyrazolo[3,4-d]pyrimidin-1-yl]piperidin-1-yl]prop-2-en-1-one](/upload/2025/4/508773e8-9ddf-4417-ba85-47f52dd5dcf6.png)
1-[(3R)-3-[4-amino-3-(4-phenoxyphenyl)pyrazolo[3,4-d]pyrimidin-1-yl]piperidin-1-yl]prop-2-en-1-one
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 4 steps
1.1: hydrazine hydrate / ethanol; water / 1 h / Heating
2.1: 4 h / 180 °C / Inert atmosphere
3.1: PS-triphenylphosphine; di-isopropyl azodicarboxylate / tetrahydrofuran / 20 °C
4.1: hydrogenchloride / 1,4-dioxane / 1 h
4.2: 2 h
With hydrogenchloride; PS-triphenylphosphine; di-isopropyl azodicarboxylate; hydrazine hydrate; In tetrahydrofuran; 1,4-dioxane; ethanol; water;
|
|
|
Multi-step reaction with 4 steps
1: triethylamine / methanol / 1 h / 50 °C
2: 5 h / 165 °C / Inert atmosphere
3: hydrogen bromide / acetic acid / 0.5 h / 0 °C
4: triethylamine / dichloromethane / 0.5 h / 0 - 20 °C
With hydrogen bromide; triethylamine; In methanol; dichloromethane; acetic acid;
|
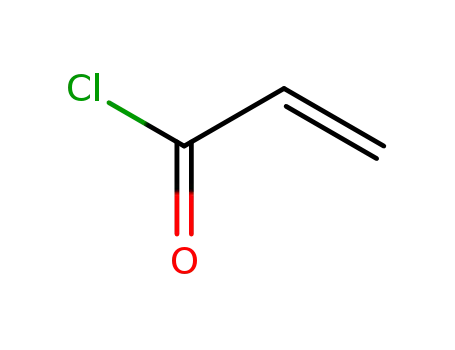
acryloyl chloride
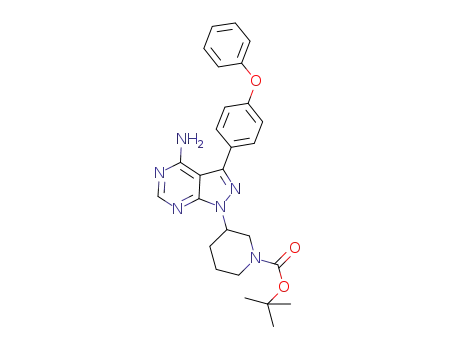
tert-butyl 3-[4-amino-3-(4-phenoxyphenyl)-1H-pyrazolo[3,4-d]pyrimidin-1-yl]piperidine-1-carboxylate
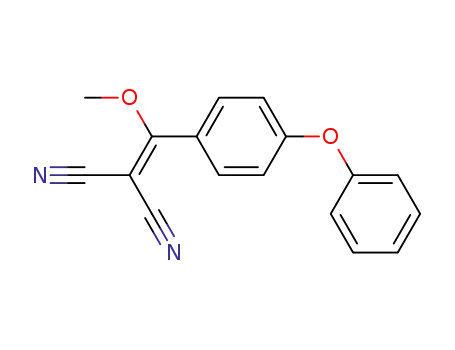
2-[(4-phenoxy-phenyl)-methoxy-methylene]-malononitrile
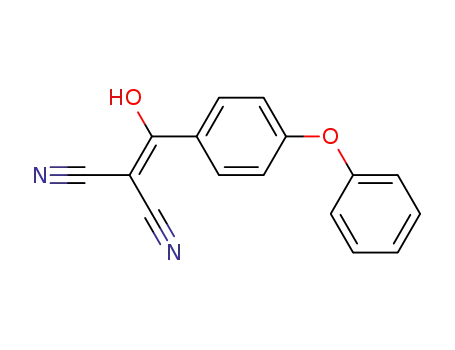
2-[hydroxy-(4-phenoxy-phenyl)-methylene]-malononitrile
CAS:112163-33-4
CAS:112-84-5
CAS:2139-90-4
CAS:906093-29-6