- +86-0533-2185556
- WhatsApp: +86 15965530500
- admin@hangyubiotech.com
Your Location:Home >Products >small-molecule inhibitor >13529-17-4
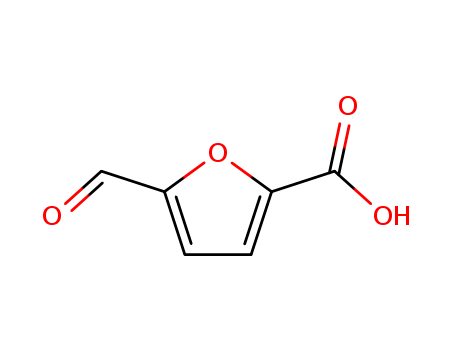

pd_meltingpoint:209 °C
Appearance:Tan solid
Purity:99%
InChI:InChI=1/C6H4O4/c7-3-4-1-2-5(10-4)6(8)9/h1-3H,(H,8,9)/p-1
-
-
Efficient oxidation of 5-hydroxymethylfu...
Electrochemical conversion of biomass-de...
Conversion of HMF into FDCA was carried ...
Utilizing sustainable biomass to partly ...
Hybrid catalysis, which combines chemo- ...
Manganese-copper layered double hydroxid...
Chloroperoxidase (CPO) catalyzes the oxi...
Here, the preparation and evaluation of ...
Green synthesis of 2,5-furandicarboxylic...
In this study, α-MoO3nanobelts were succ...
Upgradation of bio-based furans into che...
Bimetallic catalysts with Au–Pd supporte...
Defective D-CeO2@N/C@TiO2 nanospheres, e...
Environmentally friendly and efficient s...
Photocatalytic upgrading of crucial biom...
Nitrogen doped carbon spheres with wrink...
An enzyme toolbox was developed for the ...
Currently, the base-free aerobic oxidati...
A highly active and inexpensive Co–Mn mi...
5-Hydroxymethylfurfural (HMF) was quanti...
2,5-Furandicarboxylic acid (FDCA) is reg...
Furfural and 5-hydroxymethylfurfural (HM...
The reaction mechanism of 5-hydroxymethy...
Organic solvent free 5-hydroxymethylfurf...
An environment-friendly and economical r...
To construct a green chemical synthesis ...
By manipulating the reaction conditions,...
Abstract: In this study, a new kind of m...
As an effective strategic approach to pr...
The oxidation reaction of 5-hydroxymethy...
Nanoparticulate gold has emerged as a pr...
Background Fungal aryl-alcohol oxidases ...
Mg-bearing MTW silicalite zeolite, MgSi-...
A simple non-precious-metal catalyst sys...
Encapsulating noble metal nanoparticles ...
This work explores the potential-depende...
In this study, the oxidative conversion ...
Catalytic conversion of biomass or bioma...
In the present work, Pt and Pt/Sn nanopa...
The aerobic oxidation of 5-hydroxymethyl...
As a useful and renewable chemical build...
Photoelectrocatalytic (PEC) synthesis of...
A series of gold nanoparticles in the 4–...
Spinel CoMn2O4 hollow spheres were prepa...
A cryptomelane-type manganese oxide octa...
2,5-Diformylfuran (DFF) was obtained by ...
High-entropy oxides (HEOs), a new concep...
Supported Pt, Pd, and Au catalysts were ...
The catalytic conversion of 5-hydroxymet...
The Co-based electrocatalyst is among th...
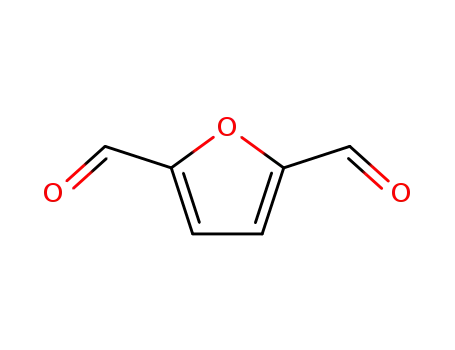
2,5-diformylfurane

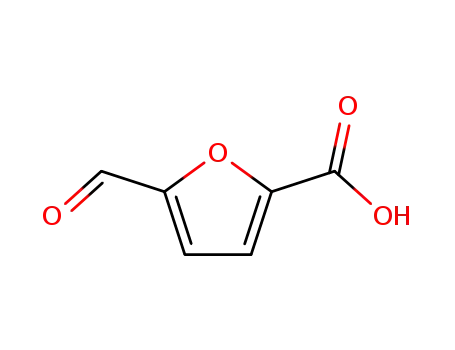
5-Formyl-2-furancarboxylic acid

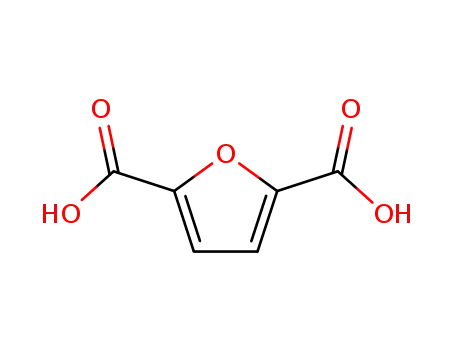
furan-2,5-dicarboxylic acid
| Conditions | Yield |
|---|---|
|
With
NOX-009; aldehyde dehydrogenase-003; catalase;
In
aq. phosphate buffer;
at 35 ℃;
for 3h;
pH=8.5;
Reagent/catalyst;
Enzymatic reaction;
|
20% 80% |
|
With
nicotinamide adenine dinucleotide;
In
aq. phosphate buffer;
at 35 ℃;
for 3h;
pH=8.5;
Concentration;
Enzymatic reaction;
|
80% 20% |
|
With
nicotinamide adenine dinucleotide;
In
aq. phosphate buffer;
at 35 ℃;
for 3h;
pH=8.5;
Concentration;
Enzymatic reaction;
|
50% 50% |
|
With
recombinant 5-hydroxymethylfurfural oxidase;
In
aq. phosphate buffer;
at 25 ℃;
for 1h;
pH=7;
Reagent/catalyst;
Enzymatic reaction;
|
|
|
With
immobilized lipase B from Candida antarctica; dihydrogen peroxide;
In
ethyl acetate; tert-butyl alcohol;
at 40 ℃;
for 24h;
Enzymatic reaction;
|
|
|
With
Fe0.6Zr0.4O2; oxygen; 1-butyl-3-methylimidazolium chloride;
at 160 ℃;
for 0.5h;
under 15001.5 Torr;
Time;
Autoclave;
|
25.6 %Chromat. 8.8 %Chromat. |
|
With
Fe0.6Zr0.4O2; oxygen; 1-butyl-3-methylimidazolium chloride;
at 160 ℃;
for 1h;
under 15001.5 Torr;
Autoclave;
|
36.0 %Chromat. 8.5 %Chromat. |
|
With
recombinant Escherichia coli cells expressing 3-succinoylsemialdehyde-pyridine dehydrogenase from Comamonastestosteroni SC1588;
In
aq. phosphate buffer;
at 30 ℃;
for 12h;
pH=7;
Enzymatic reaction;
|
77 %Chromat. 32 %Chromat. |
|
Multi-step reaction with 2 steps
1: oxygen; / water / 0.5 h / 90 °C / 7500.75 Torr / Autoclave
2: oxygen; / water / 2 h / 90 °C / 7500.75 Torr / Autoclave
With
oxygen;
In
water;
|
|
|
With
N-hydroxyphthalimide; manganese (II) acetate tetrahydrate; cobalt(II) diacetate tetrahydrate; acetic acid;
at 100 ℃;
for 1.33333h;
under 7500.75 Torr;
Reagent/catalyst;
Time;
Autoclave;
|
51.9 %Chromat. 31.4 %Chromat. |
|
With
7-hydroxy-6-methoxy-2H-1-benzopyran-2-one; 1-Benzyl-1,4-dihydronicotinamide; horseliver alcohol dehyrogenase; NAD; dihydrogen peroxide; myoglobin;
In
aq. phosphate buffer;
at 30 ℃;
for 48h;
pH=8;
Enzymatic reaction;
|
26 %Chromat. 15 %Chromat. |
C6H6O6


5-Formyl-2-furancarboxylic acid
| Conditions | Yield |
|---|---|
|
With
aluminum oxide;
In
dichloromethane;
at 180 ℃;
for 2h;
under 22502.3 Torr;
|
89% |
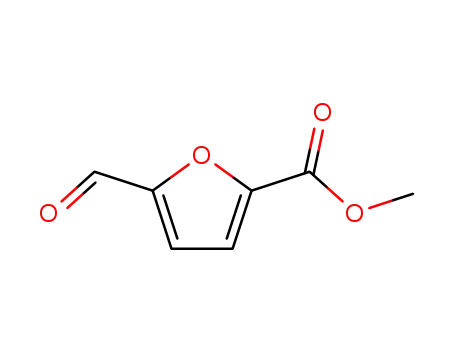
methyl 5-formylfuran-2-carboxylate
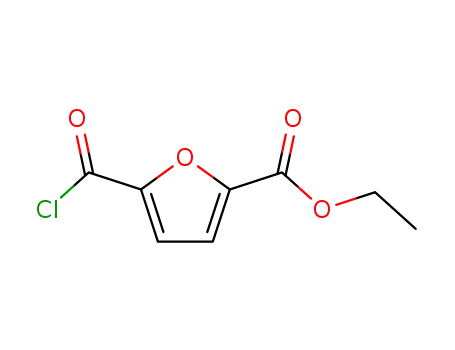
5-chlorocarbonyl-furan-2-carboxylic acid ethyl ester
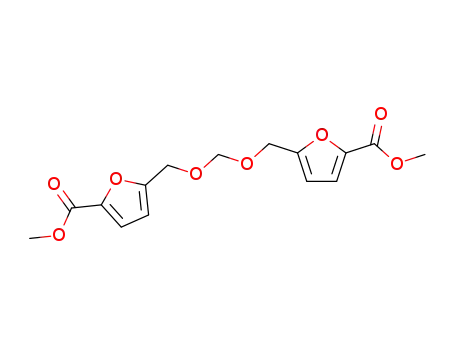
1,1-bis-(2'-methoxyfuroyl-5'-methyleneoxy)methane
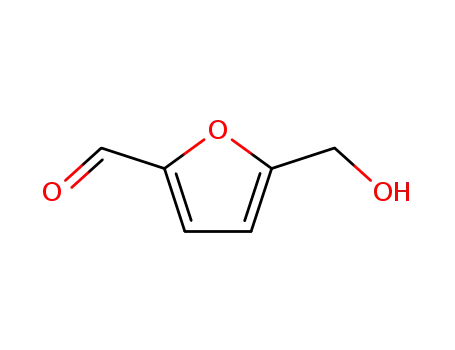
5-hydroxymethyl-2-furfuraldehyde
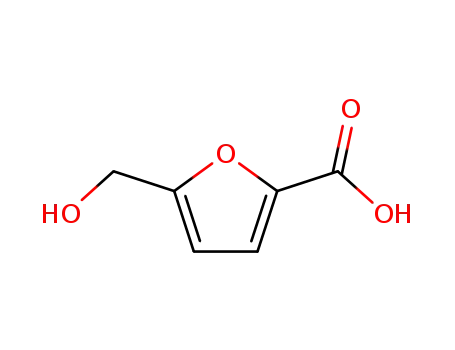
5-hydroxymethyl-furan-2-carboxylic acid

furan-2,5-dicarboxylic acid
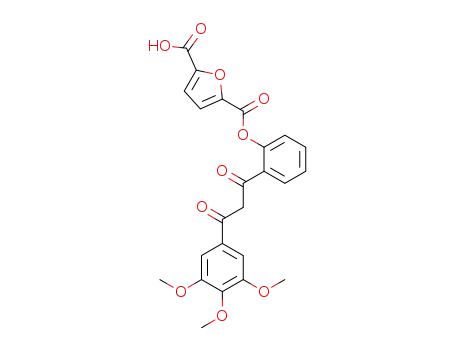
Furan-2,5-dicarboxylic acid mono-{2-[3-oxo-3-(3,4,5-trimethoxy-phenyl)-propionyl]-phenyl} ester
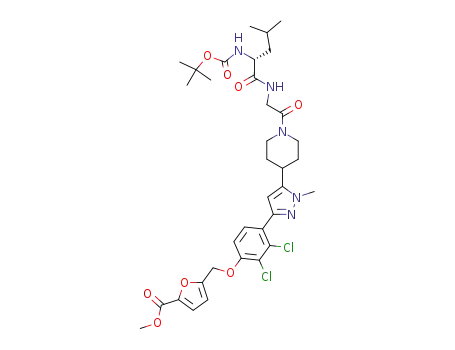
(R)-5-[4-(5-{1-[2-(2-tert-butoxycarbonylamino-4-methyl-pentanoylamino)acetyl]-piperidin-4-yl}-1-methyl-1H-pyrazol-3-yl)-2,3-dichloro-phenoxymethyl]furan-2-carboxylic acid methyl ester
CAS:112163-33-4
CAS:112-34-5
CAS:105826-92-4
CAS:105839-17-6