- +86-0533-2185556
- +86 15965530500
- admin@hangyubiotech.com
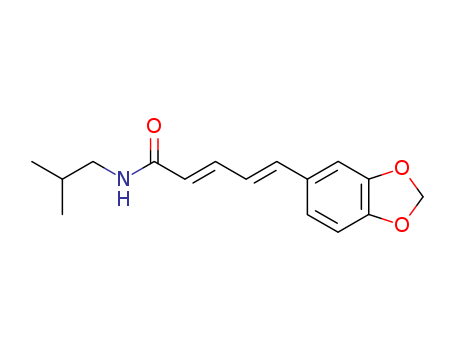

Purity:99%
|
in vitro |
in a previous study, piperlonguminine was discovered to inhibit melanin production in melanoma b16 cells stimulated with α-msh, 3-isobutyl-1-methylxanthine or protoporphyrin ix, where piperlonguminine showed stronger depigmenting efficacy. however, piperlonguminine could not alter1-oleoyl-2-acetyl-sn-glycerol-induced melanogenesis and could not affect protein kinase c-mediated melanin production. in additioin, piperlonguminine was not able to inhibit the catalytic activity of cell-free tyrosinase from melanoma b16 cells, and such effect was attributed to the inhibitory action of piperlonguminine on α-msh-induced signaling via camp to the camp responsive element binding protein [1]. |
|
in vivo |
in vivo, rats were subjected to middle cerebral artery occlusion for 1h, followed by reperfusion for 23 h. the results showed that the intraperitoneal injection of piperlonguminine pe at 2.4 mg/kg was able to produce a significant neuroprotective potential in rats with cerebral ischemia. in addition, piperlonguminine could attenuate the neurological deficit scores, brain infarct volume and brain water content, and could inhibit the activation of nf-κb and mapk [2]. |
InChI:InChI=1/C16H19NO3/c1-12(2)10-17-16(18)6-4-3-5-13-7-8-14-15(9-13)20-11-19-14/h3-9,12H,10-11H2,1-2H3,(H,17,18)
Amides are indispensable building blocks...
A series of piperic acid amides (4-24, 2...
Based on our recent findings that piperi...
A simple and practical method for the sy...
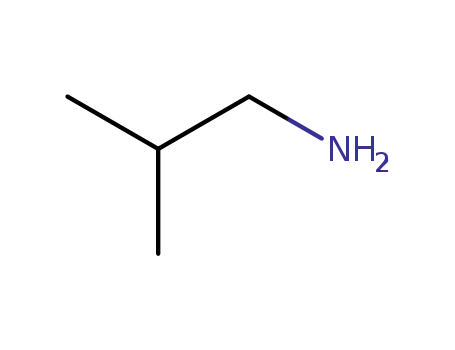
isobutylamine

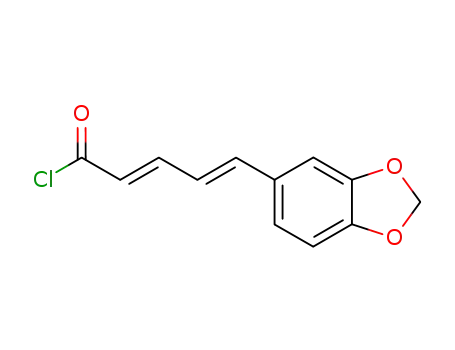
piperic acid chloride

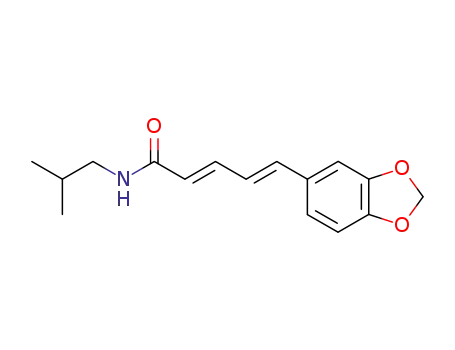
piperlonguminine
| Conditions | Yield |
|---|---|
|
With triethylamine; at 20 ℃; for 5h; Cooling with ice;
|
86% |
|
With triethylamine; In tetrahydrofuran; at 60 ℃;
|
83% |

isobutylamine

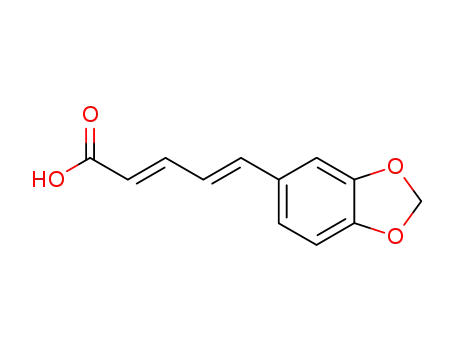
piperic acid


piperlonguminine
| Conditions | Yield |
|---|---|
|
piperic acid; With thionyl chloride; In dichloromethane; for 1h; Reflux;
isobutylamine; In dichloromethane; for 1h;
|
93% |
|
With boric acid; In toluene; for 16h; Heating;
|
91% |
|
With dmap; dicyclohexyl-carbodiimide;
|
76% |
|
With methanesulfonyl chloride; triethylamine; Yield given. Multistep reaction; 1.) CH2Cl2, 45 min., 0 deg C ; 2.) 2h, 0-25 deg C;
|
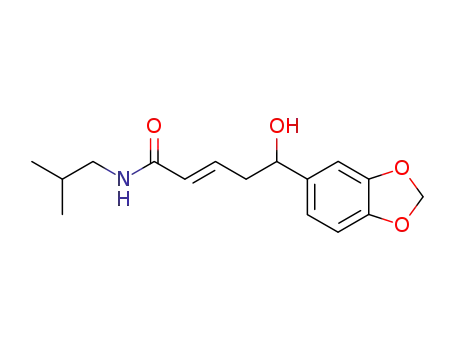
(E)-5-Benzo[1,3]dioxol-5-yl-5-hydroxy-pent-2-enoic acid isobutyl-amide

isobutylamine

piperic acid

piperic acid chloride
CAS:112163-33-4
CAS:112-84-5
CAS:478-84-2
CAS:9002-07-7