- +86-0533-2185556
- WhatsApp: +86 15965530500
- admin@hangyubiotech.com
Your Location:Home >Products >small-molecule inhibitor >324769-06-4
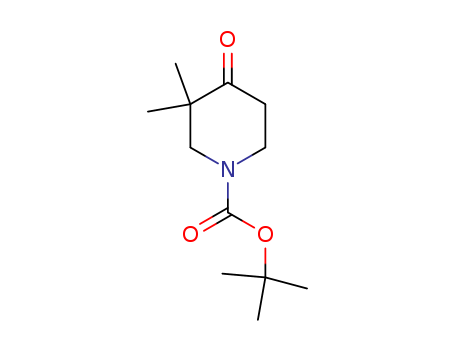

Purity:99%
|
General Description |
1-(TERT-BUTOXYCARBONYL)-3,3-DIMETHYL-4-OXOPIPERIDINE is a complex organic compound typically used in a laboratory setting due to its specific chemical properties. 1-(TERT-BUTOXYCARBONYL)-3,3-DIMETHYL-4-OXOPIPERIDINE, as its name suggests, consists of a piperidine ring, which is a saturated heterocyclic molecule. Considered a derivative of piperidine, this chemical includes additional functional groups such as a tert-butoxycarbonyl and a dimethyl group. Moreover, it also has a ketone functional group, indicated by the 4-oxo segment of the name. There is limited publicly available information regarding the specific applications or safety measures for this compound, which suggests its use is likely specialized and requires expert handling and knowledge. |
InChI:InChI=1/C12H21NO3/c1-11(2,3)16-10(15)13-7-6-9(14)12(4,5)8-13/h6-8H2,1-5H3
Post-translational modifications (PTMs) ...
A compound or its acid addition salt of ...
Disclosed are novel compounds and a meth...
The disclosure relates particularly to c...
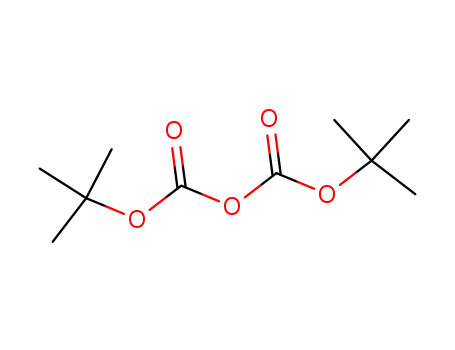
di-tert-butyl dicarbonate

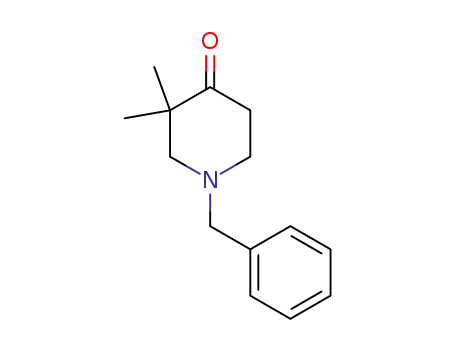
N-benzyl-3,3-dimethylpiperidine-4-one

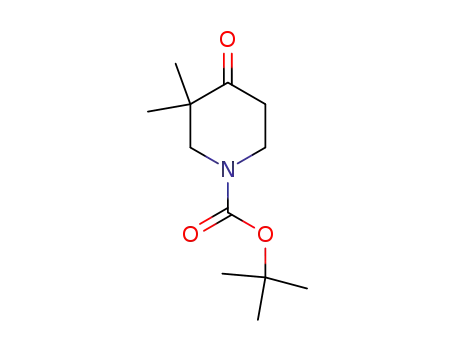
tert-butyl 3,3-dimethyl-4-oxopiperidine-1-carboxylate
| Conditions | Yield |
|---|---|
|
With
hydrogen;
palladium 10% on activated carbon;
In
methanol;
at 20 ℃;
for 16h;
under 3345.86 Torr;
|
88.5% |
|
With
hydrogen;
palladium/active carbon;
In
methanol;
at 20 ℃;
for 16h;
under 2585.81 Torr;
|
88.5% |
|
With
hydrogen;
palladium/active carbon;
In
ethanol;
at 50 ℃;
for 18h;
under 2585.81 Torr;
|
56% |
|
With
hydrogen;
palladium on activated charcoal;
In
ethanol;
|
|
|
palladium dihydroxide;
In
ethanol;
|
|
|
palladium dihydroxide;
In
ethanol;
|
|
|
With
10 wt% Pd(OH)2 on carbon; hydrogen;
In
ethanol;
at 20 ℃;
for 4h;
under 2068.65 Torr;
|
methyl iodide


tert-butyl 3,3-dimethyl-4-oxopiperidine-1-carboxylate
| Conditions | Yield |
|---|---|
|
N-tert-butyloxycarbonylpiperidin-4-one;
With
sodium hydride;
In
tetrahydrofuran;
at 0 ℃;
for 0.5h;
methyl iodide;
In
tetrahydrofuran;
at 23 ℃;
for 48h;
|
76% |
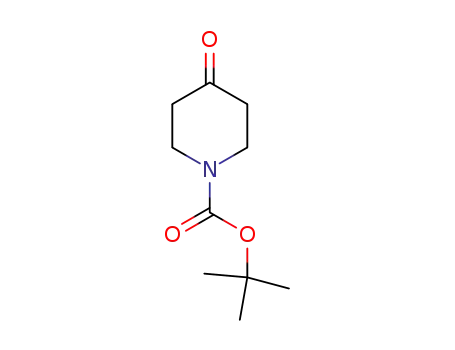
N-tert-butyloxycarbonylpiperidin-4-one

methyl iodide

di-tert-butyl dicarbonate

N-benzyl-3,3-dimethylpiperidine-4-one
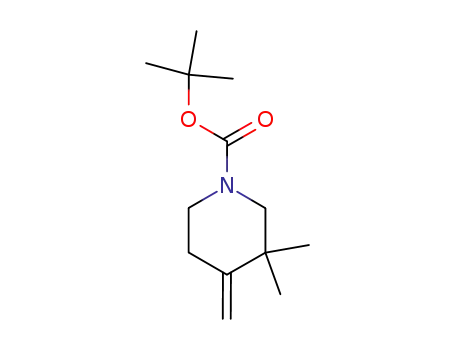
3,3-dimethyl-4-methylene-1-piperidinecarboxylic acid 1,1-dimethylethyl ester
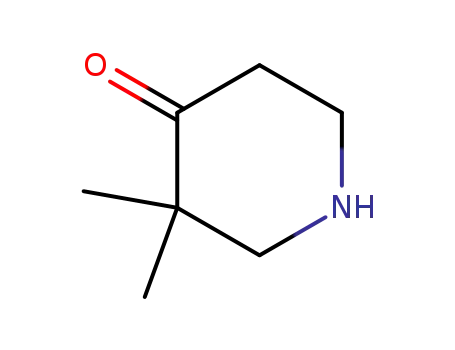
3,3-Dimethyl-piperidin-4-one
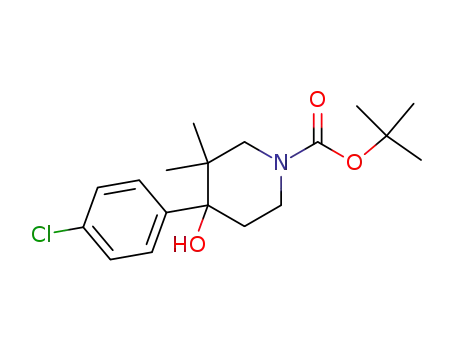
4-(4-chloro-phenyl)-4-hydroxy-3,3-dimethyl-piperidine-1-carboxylic acid tert-butyl ester
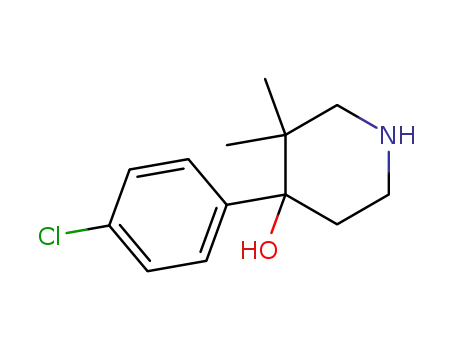
4-(4-chloro-phenyl)-3,3-dimethyl-piperidin-4-ol
CAS:112163-33-4
CAS:112-84-5
CAS:100478-25-9
CAS:35984-93-1