- +86-0533-2185556
- WhatsApp: +86 15965530500
- admin@hangyubiotech.com
Your Location:Home >Products >small-molecule inhibitor >66863-43-2
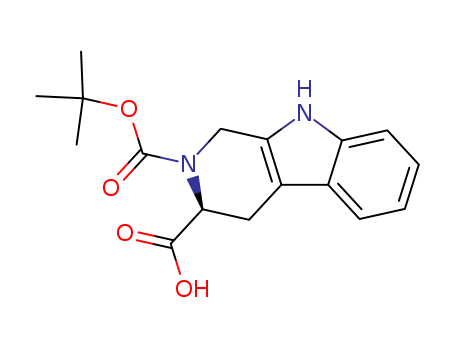

pd_meltingpoint:286-291oC
Appearance:white to light cream crystalline powder
Purity:99%
InChI:InChI=1/C17H20N2O4/c1-17(2,3)23-16(22)19-9-13-11(8-14(19)15(20)21)10-6-4-5-7-12(10)18-13/h4-7,14,18H,8-9H2,1-3H3,(H,20,21)/p-1/t14-/m0/s1
The invention discloses a hexacyclic pip...
Aminoglucose-modified pentacyclic pipera...
The invention provides a carboline ruthe...
Mitochondrial oxidative damage contribut...
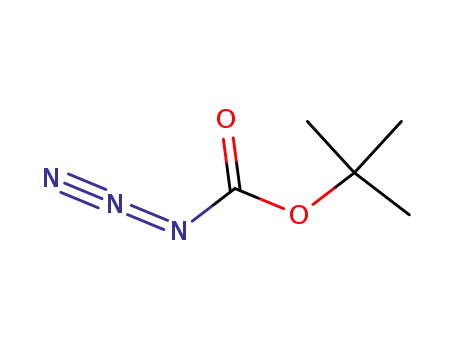
N-(tert-butyloxycarbonyl) azide

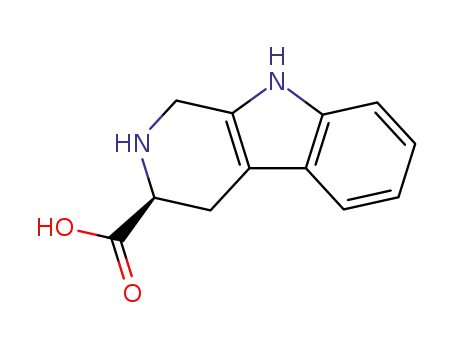
3-carboxy-1,2,3,4-tetrahydro-2-carboline

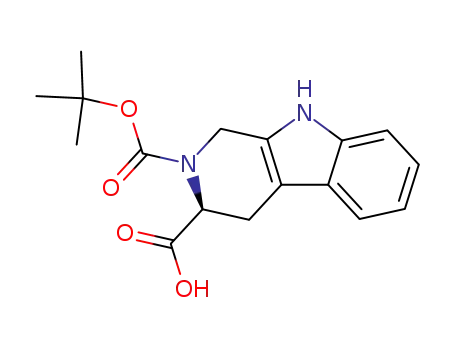
(3S)-2-tert-butoxycarbonyl-1,2,3,4-tetrahydro-beta-carboline-3-carboxylic acid
| Conditions | Yield |
|---|---|
|
With
triethylamine;
In
N,N-dimethyl-formamide;
at 20 - 40 ℃;
|
76% |
|
With
triethylamine;
In
N,N-dimethyl-formamide;
at 20 - 40 ℃;
for 104h;
|
76% |
|
With
triethylamine;
In
N,N-dimethyl-formamide;
at 20 - 40 ℃;
for 104.5h;
|
76% |
|
With
triethylamine;
In
DMF (N,N-dimethyl-formamide);
at 20 - 40 ℃;
for 104.5h;
|
76% |
|
With
triethylamine;
In
N,N-dimethyl-formamide;
at 20 ℃;
under 750.075 Torr;
Inert atmosphere;
|
76% |
|
|
50% |
|
With
triethylamine;
In
N,N-dimethyl-formamide;
at 20 - 40 ℃;
for 32.5h;
|
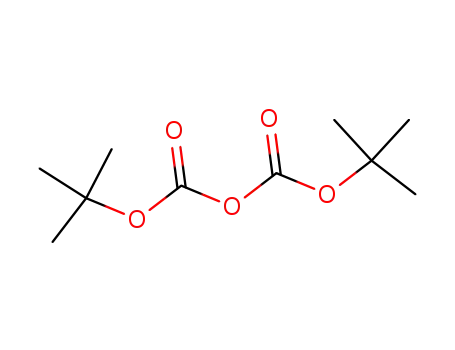
di-tert-butyl dicarbonate


3-carboxy-1,2,3,4-tetrahydro-2-carboline


(3S)-2-tert-butoxycarbonyl-1,2,3,4-tetrahydro-beta-carboline-3-carboxylic acid
| Conditions | Yield |
|---|---|
|
With
potassium carbonate;
In
tetrahydrofuran; water;
at 0 - 20 ℃;
|
97% |
|
With
potassium carbonate;
In
tetrahydrofuran; water;
at 0 - 20 ℃;
|
97% |
|
In
methanol;
at 20 ℃;
|
80% |
|
In
N,N-dimethyl-formamide;
at 0 - 20 ℃;
pH=10;
|
77% |
|
|
76% |
|
With
triethylamine;
under 750.075 Torr;
Inert atmosphere;
|
76% |
|
With
triethylamine;
In
N,N-dimethyl-formamide;
at 20 - 40 ℃;
for 104.5h;
|
76% |
|
With
triethylamine;
In
N,N-dimethyl-formamide;
at 40 ℃;
for 104h;
under 750.075 Torr;
Inert atmosphere;
|
76% |
|
With
triethylamine;
In
N,N-dimethyl-formamide;
at 0 ℃;
for 100h;
pH=10;
|
62% |
|
With
sodium hydroxide;
In
1,4-dioxane; water;
for 3h;
Reagent/catalyst;
Time;
Solvent;
Inert atmosphere;
|
61% |
|
With
triethylamine;
In
N,N-dimethyl-formamide;
at 20 ℃;
for 48h;
pH=10;
Cooling with ice;
|
25.3% |
|
In
tetrahydrofuran;
at 0 - 20 ℃;
for 19h;
|
|
|
In
N,N-dimethyl-formamide;
|
|
|
With
triethylamine;
|

N-(tert-butyloxycarbonyl) azide

3-carboxy-1,2,3,4-tetrahydro-2-carboline

di-tert-butyl dicarbonate
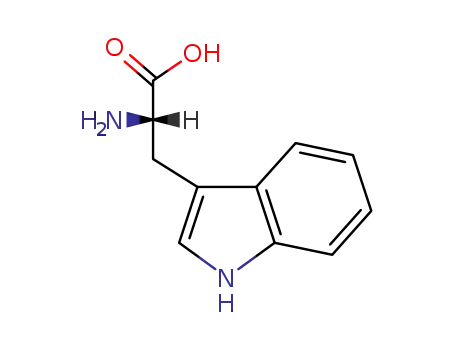
L-Tryptophan
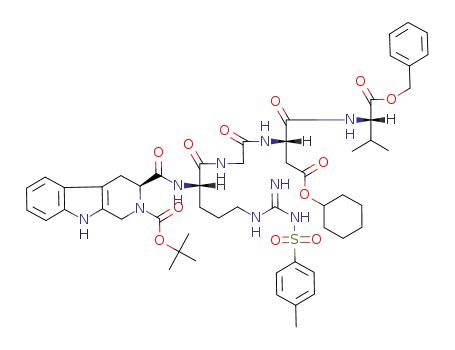
3S-2-Boc-1,2,3,4-tetrahydro-β-carboline-3-carboxyl-Arg(Tos)-Gly-Asp(OcHex)-ValOBzl
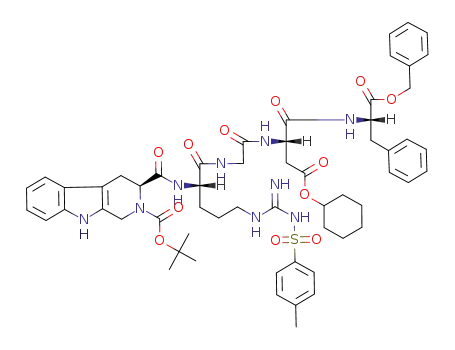
3S-2-Boc-1,2,3,4-tetrahydro-β-carboline-3-carboxyl-Arg(Tos)-Gly-Asp(OcHex)-PheOBzl
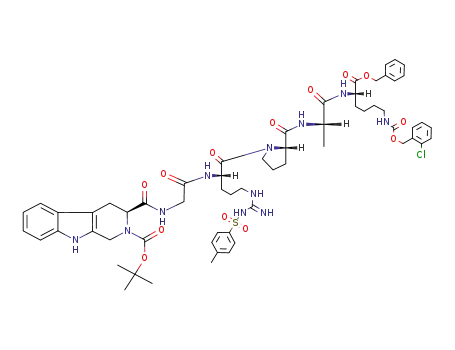
3S-(2-Boc)-1,2,3,4-tetrahydro-β-carboline-3-carboxyl-Gly-Arg(Tos)-Pro-Ala-Lys(ClZ)-OBzl
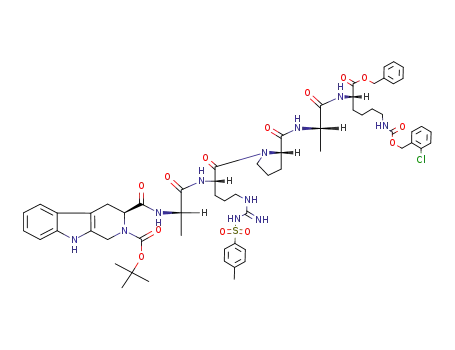
3S-(2-Boc)-1,2,3,4-tetrahydro-β-carboline-3-carboxyl-Ala-Arg(Tos)-Pro-Ala-Lys(ClZ)-OBzl
CAS:112163-33-4
CAS:112-84-5
CAS:1007-28-9
CAS:100915-96-6