- +86-0533-2185556
- WhatsApp: +86 15965530500
- admin@hangyubiotech.com
Your Location:Home >Products >small-molecule inhibitor >15401-69-1
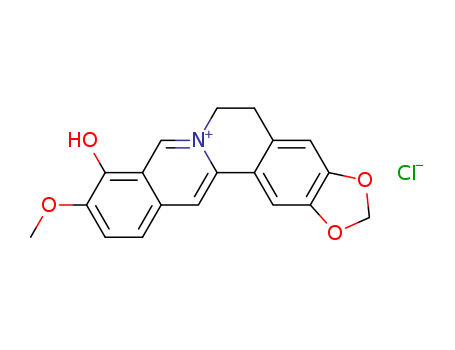

pd_meltingpoint:245℃ (DEC.)
Purity:99%
InChI:InChI=1/C19H17NO4/c1-22-16-3-2-11-6-15-13-8-18-17(23-10-24-18)7-12(13)4-5-20(15)9-14(11)19(16)21/h2-3,6-9,15,21H,4-5,10H2,1H3
The 9-demethylated derivative of the iso...
The optical signals of serum albumin (SA...
Piperazine derivatives bearing different...
Analogs of berberine 1 and related compo...
Berberine (1) is an alkaloid used widely...
Berberine, a naturally occurring compoun...
Aminothiazolyl berberine derivatives as ...
The paper describes the synthesis of new...
A series of natural berberine-derived ni...
Two novel Pt(ii) complexes, [Pt(B-TFA)Cl...
A variety of heterocyclic nitrogen cores...
A series of novel Schiff base-bridged te...
To alter its hydrophobicity, a series of...
Berberine is a bioactive isoquinoline al...
The novel target products were synthesiz...
The development of small molecule fluore...
-
Abstract: Berberine is a naturally occur...
Principal differences in the interaction...
The discovery and structural optimizatio...
A series of new 9-O-substituted berberin...
Isoquinoline alkaloids possess versatile...
The present manuscript reports a lucrati...
Natural berberine-hybridized benzimidazo...
Six novel berberine dimers (3a-f) were s...
A series of novel berberine-based imidaz...
Taking berberine (BBR) as the lead, 23 n...
A series of novel berberine-benzimidazol...
This communication describes the facile ...
Structural modification of active natura...
Liquid chromatography/atmospheric pressu...
-
Epileptic seizures are characterized by ...
Piperazine moieties with disubstituted N...
The invention discloses a preparation me...
The invention discloses a novel berberin...
Berberine (BBR), a kind of quaternary am...
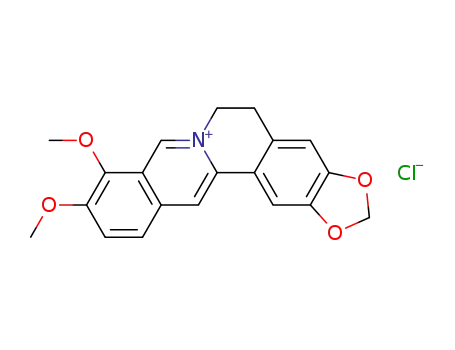
berberine chloride

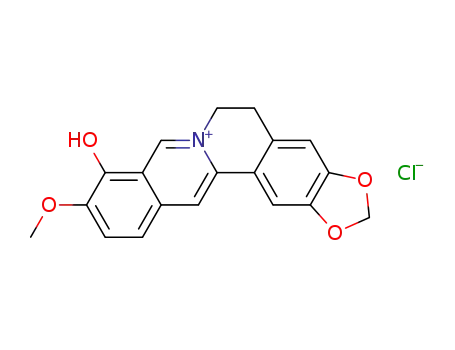
berberrubine chloride
| Conditions | Yield |
|---|---|
|
at 180 ℃;
for 0.333333h;
|
98% |
|
In
N,N-dimethyl-formamide;
at 160 ℃;
|
94% |
|
at 190 ℃;
for 0.333333h;
|
93.92% |
|
at 190 ℃;
for 0.333333h;
|
93.92% |
|
at 190 ℃;
for 3h;
|
93.9% |
|
In
N,N-dimethyl-formamide;
for 0.25h;
Microwave irradiation;
|
93% |
|
In
N,N-dimethyl-formamide;
for 0.25h;
Reflux;
Microwave irradiation;
|
92% |
|
berberine chloride;
at 180 - 190 ℃;
With
hydrogenchloride;
In
ethanol;
|
92.4% |
|
at 190 ℃;
under 20 - 30 Torr;
|
90% |
|
at 190 ℃;
for 0.25h;
under 20 - 30 Torr;
|
90% |
|
at 190 ℃;
for 0.25h;
under 20 - 30 Torr;
|
90% |
|
In
1-methyl-pyrrolidin-2-one;
at 150 - 155 ℃;
for 3h;
Temperature;
|
90% |
|
at 190 ℃;
for 0.75h;
|
89.8% |
|
at 190 ℃;
for 2h;
under 20 Torr;
|
88.2% |
|
at 190 ℃;
for 0.25h;
under 20 Torr;
|
88.4% |
|
at 190 ℃;
for 2h;
under 20 Torr;
|
88.2% |
|
at 190 ℃;
for 0.5h;
under 20 Torr;
|
88.4% |
|
at 190 ℃;
under 20 - 30 Torr;
|
88.7% |
|
at 170 ℃;
for 1h;
under 750.075 Torr;
|
88.3% |
|
at 195 ℃;
under 30 Torr;
|
87.5% |
|
With
water;
at 190 ℃;
for 1h;
under 0.750075 Torr;
|
85.9% |
|
at 190 ℃;
for 0.666667h;
under 20 - 30 Torr;
|
85% |
|
at 190 ℃;
for 0.666667h;
under 20 - 30 Torr;
|
85% |
|
at 190 ℃;
for 0.666667h;
under 20 - 30 Torr;
|
85% |
|
at 190 ℃;
for 0.666667h;
under 20 - 30 Torr;
|
85% |
|
With
hydrogenchloride;
In
ethanol;
at 190 ℃;
for 0.25h;
under 20 Torr;
|
84.6% |
|
In
neat (no solvent);
at 195 - 200 ℃;
under 20 - 30 Torr;
Temperature;
|
81% |
|
With
hydrogenchloride;
In
ethanol;
at 190 ℃;
for 0.333333h;
|
80% |
|
at 195 - 210 ℃;
under 30 - 40 Torr;
|
80% |
|
berberine chloride;
at 195 - 210 ℃;
With
hydrogenchloride;
In
ethanol; water;
|
80% |
|
for 1h;
under 30 - 40 Torr;
|
80% |
|
at 190 ℃;
|
79% |
|
With
hydrogenchloride;
In
ethanol;
at 190 ℃;
for 0.75h;
under 20 Torr;
|
79% |
|
at 190 ℃;
Pyrolysis;
Inert atmosphere;
|
79% |
|
at 190 ℃;
for 0.5h;
under 20 - 30 Torr;
|
79% |
|
at 205 ℃;
for 2h;
under 11.2511 Torr;
|
77% |
|
at 195 - 210 ℃;
for 0.5h;
under 20 - 30 Torr;
Pyrolysis;
|
74% |
|
at 190 ℃;
for 0.25h;
under 20 - 30 Torr;
|
69% |
|
for 0.0833333h;
Irradiation;
|
67% |
|
at 190 ℃;
for 0.75h;
under 10 - 15 Torr;
|
65% |
|
In
N,N-dimethyl-formamide;
at 190 ℃;
for 2h;
|
65% |
|
at 195 ℃;
for 0.666667h;
regioselective reaction;
|
62.5% |
|
at 190 ℃;
|
60% |
|
at 190 ℃;
for 0.25h;
|
60% |
|
at 190 ℃;
for 0.5h;
|
60% |
|
at 195 - 210 ℃;
for 0.25h;
under 20 - 30 Torr;
|
60% |
|
at 190 ℃;
for 0.5h;
Vacuum;
|
60% |
|
at 190 ℃;
for 0.333333h;
under 20 - 30 Torr;
Inert atmosphere;
|
60% |
|
at 190 - 210 ℃;
for 0.5h;
under 30 - 40 Torr;
|
60% |
|
at 190 ℃;
for 0.5h;
|
60% |
|
With
hydrogenchloride;
at 190 ℃;
for 2h;
Inert atmosphere;
|
50.7% |
|
at 190 ℃;
for 0.25h;
under 20 - 30 Torr;
|
660 mg |
|
Heating;
|
|
|
at 190 ℃;
for 0.5h;
|
60 %Spectr. |
|
at 190 ℃;
for 0.25h;
under 20 - 30 Torr;
|
|
|
berberine chloride;
at 195 ℃;
for 0.25h;
With
hydrogenchloride;
In
ethanol;
at 20 ℃;
for 1h;
|
|
|
at 190 ℃;
for 1.5h;
under 30 Torr;
|
8.37 g |
|
Multi-step reaction with 2 steps
1: 0.25 h / 190 °C / 30 Torr
2: hydrogenchloride / ethanol / 20 °C
With
hydrogenchloride;
In
ethanol;
|
|
|
at 190 ℃;
for 0.666667h;
under 20 - 30 Torr;
|
|
|
With
piperidine; water;
In
ethanol;
Reagent/catalyst;
Solvent;
Reflux;
|
|
|
In
N,N-dimethyl-formamide;
at 190 ℃;
Reflux;
|
|
|
at 190 ℃;
for 0.5h;
under 20 - 30 Torr;
|
|
|
With
hydrogenchloride;
In
ethanol; water;
at 190 ℃;
|
|
|
With
mercury;
at 190 ℃;
|
|
|
at 190 ℃;
|
|
|
at 180 - 200 ℃;
for 1.25h;
under 20 - 30 Torr;
|
|
|
Multi-step reaction with 2 steps
1: 0.5 h / 185 °C / 20 - 30 Torr
2: hydrogenchloride / ethanol; water / 8 h / 20 °C
With
hydrogenchloride;
In
ethanol; water;
|
|
|
berberine chloride;
at 195 - 205 ℃;
for 0.5h;
With
hydrogenchloride;
In
ethanol;
at 20 ℃;
for 1h;
|
|
|
With
hydrogenchloride;
In
ethanol;
at 20 - 190 ℃;
for 1.33333h;
|
|
|
at 190 ℃;
|
berberrubine


berberrubine chloride
| Conditions | Yield |
|---|---|
|
With
hydrogenchloride;
In
ethanol; water;
at 20 ℃;
for 8h;
|
98% |
|
With
hydrogenchloride;
In
chloroform;
Ambient temperature;
|
94.1% |
|
With
hydrogenchloride;
In
ethanol; water;
pH=5 - 6;
|
82% |
|
With
hydrogenchloride;
In
water;
|
|
|
With
hydrogenchloride;
In
ethanol;
at 20 ℃;
|

berberrubine

berberine chloride
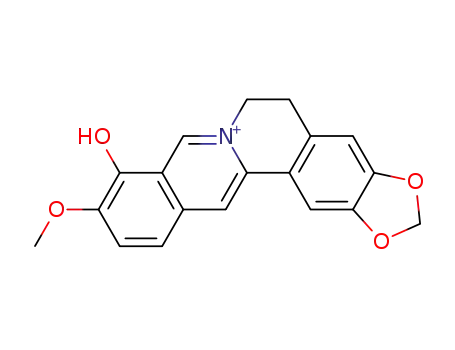
berberrubine
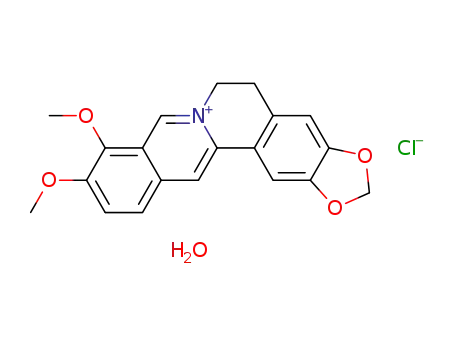
berberine chloride hydrate
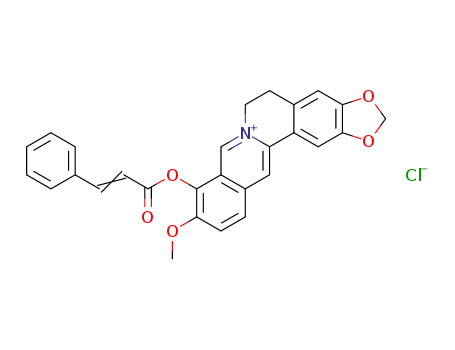
2,3-methenedioxy-9-cinnamate-10-methoxyprotoberberine chloride
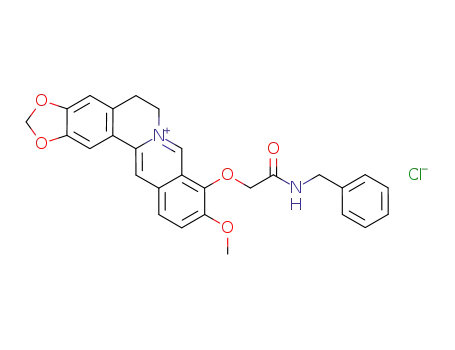
C28H25N2O5(1+)*Cl(1-)
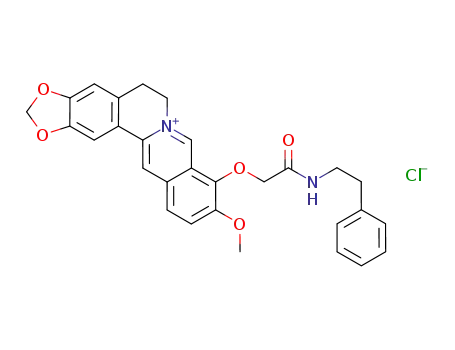
C29H27N2O5(1+)*Cl(1-)
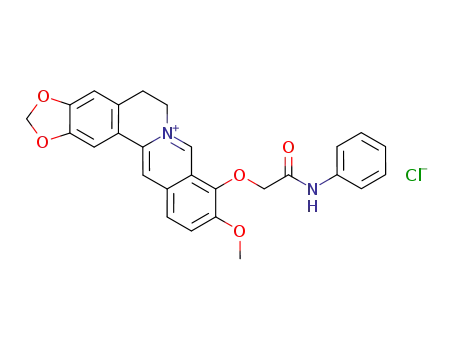
C27H23N2O5(1+)*Cl(1-)
CAS:118685-33-9
CAS:6138-41-6
CAS:3333-15-1
CAS:3697-38-9