- +86-0533-2185556
- WhatsApp: +86 15965530500
- admin@hangyubiotech.com
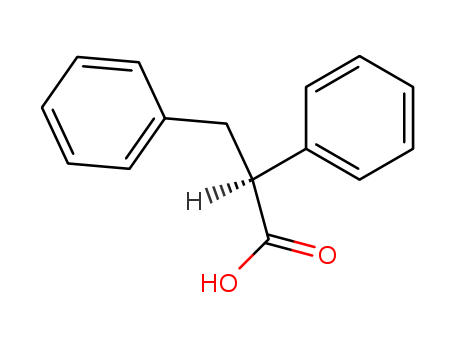

pd_meltingpoint:89°C
Purity:99%
|
Synthesis Reference(s) |
Journal of the American Chemical Society, 78, p. 4942, 1956 DOI: 10.1021/ja01600a035 |
InChI:InChI=1/C15H14O2/c16-15(17)14(13-9-5-2-6-10-13)11-12-7-3-1-4-8-12/h1-10,14H,11H2,(H,16,17)
Enantioselective hydrogenations of α,β-u...
We have developed an electrocatalytic as...
The effects of the quinoline ring modifi...
Shape-controlled metal nanocrystals poss...
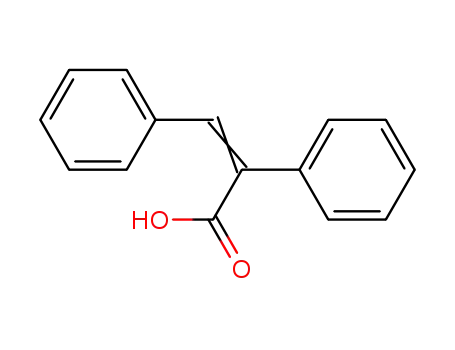
2,3-diphenylacrylic acid

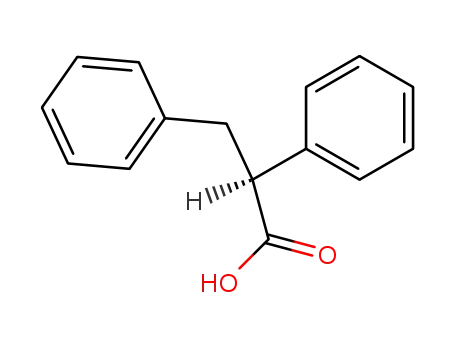
(2S)-2,3-diphenylpropanoic acid

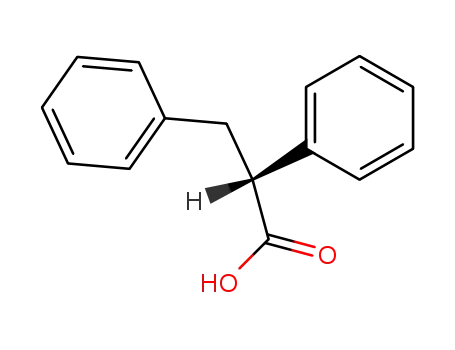
(R)-2,3-diphenylpropanoic acid
| Conditions | Yield |
|---|---|
|
With bis(norbornadiene)rhodium(l)tetrafluoroborate; (S(p),S(p))-2,2''-bis(diphenylphosphino)-1,1''-biferrocene; hydrogen; In methanol; at 25 ℃; for 14.5h; under 60006 Torr; optical yield given as %ee; enantioselective reaction; Autoclave;
|
|
|
With 5% Pd(II)/C(eggshell); Cinchonidin; In 1,4-dioxane; water; at 22.84 ℃; optical yield given as %ee; enantioselective reaction; Kinetics;
|
|
|
With 5% Pd(II)/C(eggshell); Cinchonin; In 1,4-dioxane; water; at 22.84 ℃; optical yield given as %ee; enantioselective reaction; Kinetics;
|
|
|
With 5% Pd(II)/C(eggshell); hydrogen; cinchonidine; benzylamine; In 1,4-dioxane; water; at 49.84 ℃; optical yield given as %ee; enantioselective reaction;
|
|
|
With 5%-palladium/activated carbon; hydrogen; cinchonine; In 1,4-dioxane; water; at 23 ℃; under 1500.15 Torr; optical yield given as %ee; enantioselective reaction;
|
|
|
With palladium on activated charcoal; hydrogen; cinchonidine; benzylamine; In 1,4-dioxane; at 22.84 ℃; under 7500.75 Torr; Overall yield = 100%; Optical yield = 82%ee; enantioselective reaction;
|
|
|
With cinchonidine; 5% Pd/CNT; hydrogen; benzylamine; In 1,4-dioxane; water; under 750.075 Torr; Reagent/catalyst; enantioselective reaction; Catalytic behavior;
|
75 % ee |
|
With bis(norbornadiene)rhodium(l)tetrafluoroborate; (R)-1-[2-(2'-diphenylphosphinophenyl)-ferrocenyl]ethyldi[bis-(3,5-trifluoromethyl)phenyl]phosphine; hydrogen; In methanol; at 20 ℃; for 19h; under 37503.8 Torr; Inert atmosphere; Autoclave;
|
82% ee |
|
With bis(norbornadiene)rhodium(l)tetrafluoroborate; (Sp)-2-{(R)-1-bis[3,5-bis(trifluoromethyl)phenyl]phosphinoethyl}-(Sp)-2″-diphenylphosphino-1,1″-biferrocene; hydrogen; In methanol; at 20 ℃; for 19h; under 37503.8 Torr; Inert atmosphere; Autoclave;
|
80% ee |
|
With hydrogen; benzylamine; In 1,4-dioxane; water; at 22.84 ℃; under 760.051 Torr; enantioselective reaction;
|
80 % ee |
|
With 5%-palladium/activated carbon; hydrogen; benzylamine; Cinchonidin; In 1,4-dioxane; water; at 22.84 ℃; Reagent/catalyst; enantioselective reaction;
|
58 % ee |
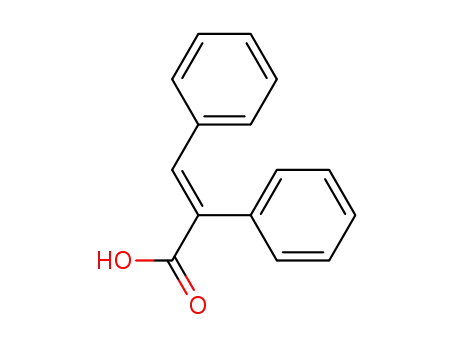
(E)-2,3-diphenylpropenoic acid


(2S)-2,3-diphenylpropanoic acid


(R)-2,3-diphenylpropanoic acid
| Conditions | Yield |
|---|---|
|
With hydrogen; Cinchonidin; In water; N,N-dimethyl-formamide; at 24.9 ℃; Product distribution; conversion dependence of the optical yield;
|
|
|
With hydrogen; cinchonidine; In methanol; at 24.9 ℃; for 0.333333h; under 760 Torr; Product distribution; variation of cinchona alkaloids on Pd/TiO2 catalyst and solvent;
|
|
|
With hydrogen; cinchonidine; In water; N,N-dimethyl-formamide; at 24.9 ℃; under 760 Torr;
|
|
|
With hydrogen; diacetato[(R)-(+)-2,2'-bis(diphenylphosphino)-5,5',6,6',7,7',8,8'-octahydro-1,1'-binaphthyl]ruthenium(II); In methanol; at 60 ℃; for 61h; under 20520 Torr; Yield given. Yields of byproduct given. Title compound not separated from byproducts;
|
|
|
With hydrogen; benzylamine; chinchonidine-modified Pd; In 1,4-dioxane; water; at 24.85 ℃; under 750.06 Torr;
|
|
|
With hydrogen; palladium; In 1,4-dioxane; water; at 24.85 ℃;
|
|
|
With hydrogen; cinchonidine-modified Pd; In 1,4-dioxane; water; at 19.85 ℃; under 0.1 Torr; Further Variations:; Pressures; Product distribution;
|
|
|
With hydrogen; [Rh(cycloocta-1,5-diene){(S,S,S)-dipof}]CF3SO3; In methanol; at 39.85 ℃; for 24h; under 15001.2 Torr; Further Variations:; Catalysts; Product distribution;
|
|
|
With hydrogen; benzylamine; Cinchonidin; palladium; In 1,4-dioxane; water; at 22 - 24 ℃; Further Variations:; Reagents; Product distribution; atmospheric pressure;
|
|
|
With bis(1,5-cyclooctadiene)rhodium(I) tetrafluoroborate; phosphoramidite based on chiral-3,3'-dimethylbinol; hydrogen; tris-(o-tolyl)phosphine; In water; isopropyl alcohol; at 60 ℃; for 16h; under 18751.5 Torr; Title compound not separated from byproducts;
|
|
|
With 5%-palladium/activated carbon; hydrogen; cinchonidine; benzylamine; In 1,4-dioxane; water; at 23 ℃; under 760.051 Torr; optical yield given as %ee; enantioselective reaction;
|
|
|
With palladium/alumina; hydrogen; benzylamine; (8R,9S)-cinchonine; In water; N,N-dimethyl-formamide; at -0.16 ℃; under 750.075 Torr; optical yield given as %ee;
|
|
|
With 10% Pd/Al2O3; hydrogen; cinchonidine; benzylamine; In water; N,N-dimethyl-formamide; at 24.84 ℃; under 750.075 Torr; optical yield given as %ee; enantioselective reaction;
|
|
|
With 10% Pd/Al2O3; hydrogen; cinchonine; benzylamine; In water; N,N-dimethyl-formamide; at 24.84 ℃; under 750.075 Torr; optical yield given as %ee; enantioselective reaction;
|
|
|
With 5% Pd/Al2O3; hydrogen; cinchonidine; benzylamine; In water; N,N-dimethyl-formamide; at 1.84 ℃; under 750.075 Torr; optical yield given as %ee; enantioselective reaction;
|
|
|
With C51H54IrNOP; hydrogen; caesium carbonate; In methanol; at 45 ℃; for 12h; under 4560.31 Torr; optical yield given as %ee; enantioselective reaction;
|
|
|
With bis(norbornadiene)rhodium(l)tetrafluoroborate; (S)-1-{(SFc)-2-[2-(diphenylphosphino)phenyl]ferrocenyl}ethylbis[3,5-bis-(trifluoromethyl)phenyl]phosphine; hydrogen; In methanol; at 20 ℃; for 19h; under 38002.6 Torr; Reagent/catalyst; Autoclave;
|
89 % ee |
|
Multi-step reaction with 4 steps
1: thionyl chloride / 4 - 42 °C
2: diisobutylaluminium hydride / tetrahydrofuran / 2 h / -70 - -10 °C
3: Dess-Martin periodane / dichloromethane / 2.5 h / -10 °C
4: aldehyde dehydrogenase from bovine lens; NADH; ene-reductase from Gluconobacter oxydans / aq. phosphate buffer / 30 °C / pH 7 / Enzymatic reaction
With thionyl chloride; aldehyde dehydrogenase from bovine lens; ene-reductase from Gluconobacter oxydans; diisobutylaluminium hydride; Dess-Martin periodane; NADH; In tetrahydrofuran; aq. phosphate buffer; dichloromethane;
|
|
|
Multi-step reaction with 4 steps
1: thionyl chloride / 4 - 42 °C
2: diisobutylaluminium hydride / tetrahydrofuran / 2 h / -70 - -10 °C
3: Dess-Martin periodane / dichloromethane / 2.5 h / -10 °C
4: aldehyde dehydrogenase from bovine lens; NADH; ene-reductase from Bacillus subtilis / aq. phosphate buffer / 30 °C / pH 7 / Enzymatic reaction
With thionyl chloride; aldehyde dehydrogenase from bovine lens; ene-reductase from Bacillus subtilis; diisobutylaluminium hydride; Dess-Martin periodane; NADH; In tetrahydrofuran; aq. phosphate buffer; dichloromethane;
|
|
|
With 5%-palladium/activated carbon; hydrogen; benzylamine; Cinchonidin; In 1,4-dioxane; water; at 22.84 ℃;
|
83 % ee |
|
With 4.92 % Pd/C; hydrogen; cinchonidine; benzylamine; In 1,4-dioxane; at 23 ℃; Reagent/catalyst; Optical yield = 70 %ee; enantioselective reaction;
|
|
|
With hydrogen; at -0.16 ℃; Temperature; Optical yield = 80 %ee; enantioselective reaction;
|
|
|
(E)-2,3-diphenylpropenoic acid; With 5%-palladium/activated carbon; hydrogen; benzylamine; Cinchonidin; In 1,4-dioxane; water; at 22.84 - 79.84 ℃; for 2h; under 750.075 Torr;
With hydrogenchloride; In water; enantioselective reaction;
|
82 % ee |
|
(E)-2,3-diphenylpropenoic acid; With 5%-palladium/activated carbon; hydrogen; benzylamine; (+)-cinchonine; In 1,4-dioxane; water; at 22.84 ℃; under 750.075 Torr;
With hydrogenchloride; enantioselective reaction;
|
54 % ee |
|
With hydrogen; benzylamine; In 1,4-dioxane; water; at 20 ℃; for 2h; under 76.0051 Torr; Overall yield = 49.8 %; enantioselective reaction;
|
17 % ee |
|
With hydrogen; benzylamine; In 1,4-dioxane; water; at 20 ℃; for 2h; under 76.0051 Torr; Overall yield = 48.4 %; enantioselective reaction;
|
9 % ee |
|
With hydrogen; benzylamine; In 1,4-dioxane; water; at 22.84 ℃; under 750.075 Torr; Reagent/catalyst;
|
71 % ee |
|
With hydrogen; benzylamine; In 1,4-dioxane; water; at 22.84 ℃; under 750.075 Torr; Reagent/catalyst;
|
36 % ee |
|
With palladium on activated charcoal; hydrogen; benzylamine; (1S,3R,4S,8S,9S)-9-hydroxy-cinchonane; In 1,4-dioxane; at 20 ℃; enantioselective reaction; Electrochemical reaction;
|
19 % ee |
|
With palladium on activated charcoal; hydrogen; benzylamine; Cinchonidin; In 1,4-dioxane; at 20 ℃; Reagent/catalyst; enantioselective reaction; Electrochemical reaction;
|
44 % ee |
|
With C56H36F12FeO10P2Ru4; hydrogen; In ethanol; toluene; at 100 ℃; for 24h; under 37503.8 Torr; Reagent/catalyst; enantioselective reaction; Autoclave;
|
83 % ee |
|
With C52H52FeO10P2Ru4; hydrogen; In ethanol; toluene; at 100 ℃; for 24h; under 37503.8 Torr; Reagent/catalyst; enantioselective reaction; Autoclave;
|
57 % ee |
|
With 5%-palladium/activated carbon; hydrogen; benzylamine; Cinchonidin; In 1,4-dioxane; water; at 22.84 ℃; for 2h; under 750.075 Torr; Reagent/catalyst; enantioselective reaction; Kinetics;
|
83 % ee |
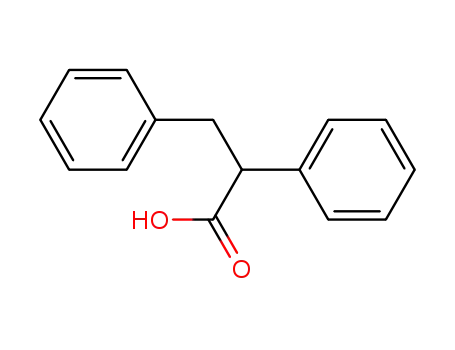
2,3-diphenylpropanoic acid

(E)-2,3-diphenylpropenoic acid
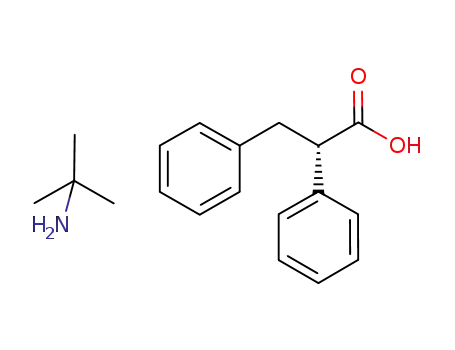
(S)-2,3-diphenylpropionic acid tert-butylammonium salt

2,3-diphenylacrylic acid
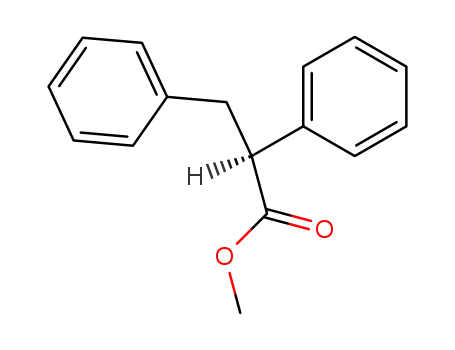
(S)-2,3-diphenyl-propionic acid methyl ester
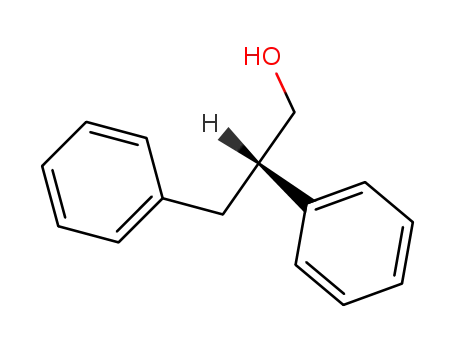
(S)-2,3-diphenyl-1-propanol
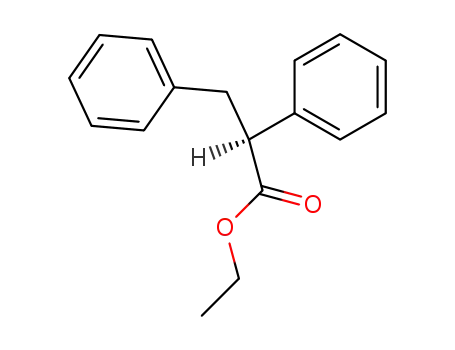
(S)-2,3-Diphenyl-propionic acid ethyl ester
CAS:112163-33-4
CAS:112-34-5
CAS:1016731-24-0
CAS:15401-69-1