- +86-0533-2185556
- WhatsApp: +86 15965530500
- admin@hangyubiotech.com
Your Location:Home >Products >API >17220-38-1
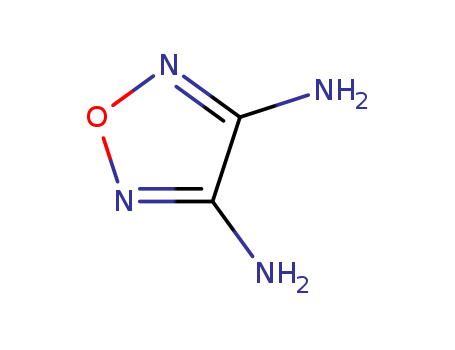

pd_meltingpoint:178-183 °C
Appearance:white to slightly beige crystalline powder
Purity:99%
InChI:InChI=1/C4H6N2O/c5-3-1-7-2-4(3)6/h1-2H,5-6H2
Highly energetic 3,4-di(nitramino)furaza...
On the basis of a design strategy that r...
Azoxyfurazan derivatives based on the tr...
Energetic compounds with fused tetracycl...
Two energetic N-trinitroethyl-substitute...
Diaminofurazan (1) was synthesized from ...
-
The invention relates to a tetrazine-fur...
The invention discloses a kilogram-grade...
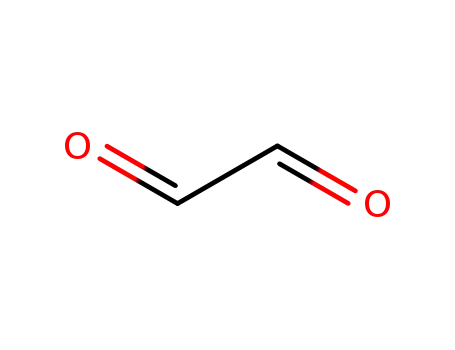
Glyoxal

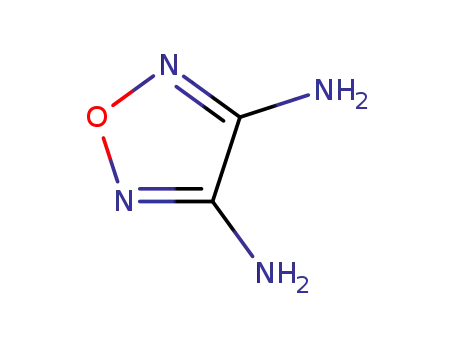
3,4-diaminofurazan
| Conditions | Yield |
|---|---|
|
With hydroxylamine hydrochloride; urea; sodium hydroxide; In water; at 105 ℃; for 2h;
|
67% |
|
Glyoxal; With hydroxylamine hydrochloride; sodium hydroxide; In water; at 108 ℃; for 7h; Cooling with ice;
With urea; In water; at 100 - 110 ℃; for 24h; Temperature;
|
50% |
|
With hydroxylamine hydrochloride; urea; sodium hydroxide; In water; at 110 ℃;
|
45% |
|
With hydroxylamine hydrochloride; urea; at 102 - 108 ℃;
|
|
|
With hydroxylamine hydrochloride; urea; at 102 - 108 ℃;
|
|
|
With hydroxylamine hydrochloride; urea; sodium hydroxide; at 125 ℃; for 15h;
|
|
|
With hydroxylamine hydrochloride; urea; sodium hydroxide; at 105 ℃; for 15h;
|
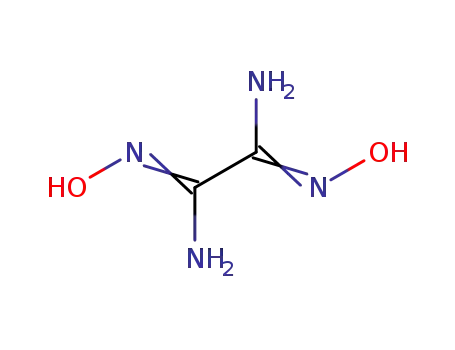
diaminoglyoxime


3,4-diaminofurazan
| Conditions | Yield |
|---|---|
|
With potassium hydroxide; at 170 - 180 ℃; for 2h;
|
70% |
|
With potassium hydroxide; for 0.333333h; microwave irradiation;
|
70% |
|
With potassium hydroxide; In ethylene glycol; at 120 - 170 ℃; for 1h; Product distribution / selectivity;
|
52% |
|
In ethylene glycol; at 165 ℃; for 0.5h; Product distribution / selectivity;
|
52% |
|
With potassium hydroxide; at 170 ℃; for 2h;
|
50% |
|
With sodium hydroxide; at 165 ℃;
|
|
|
With sodium hydroxide; In N,N-dimethyl-formamide; for 4h; Ambient temperature;
|
|
|
With potassium hydroxide; at 180 ℃;
|
|
|
at 165 ℃; for 0.5h; Product distribution / selectivity; Neat (no solvent);
|
|
|
With potassium hydroxide; In water;
|

diaminoglyoxime
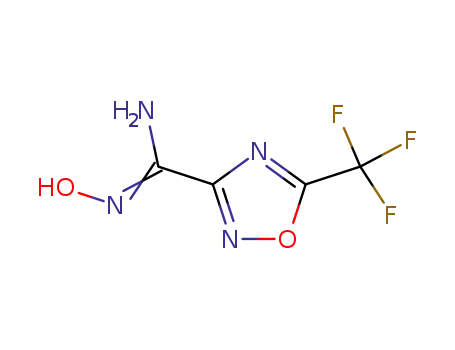
5-trifluoromethyl-1,2,4-oxadiazole-3-carboxyamidoxime
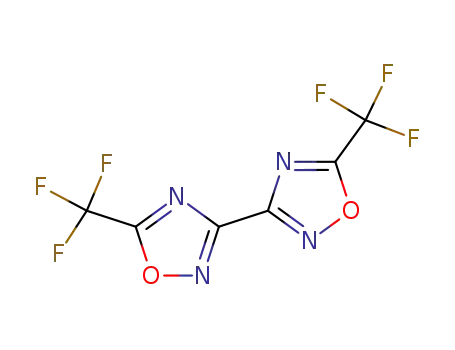
3,3’-bi-(5-trifluoromethyl-1,2,4-oxadiazole)
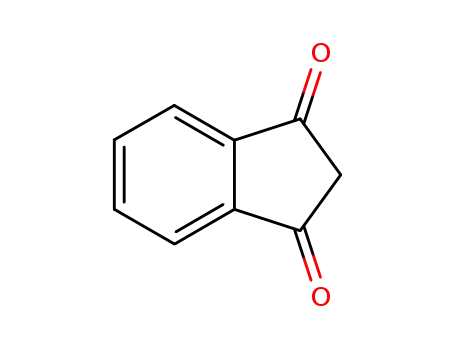
1H-indene-1,3(2H)-dione
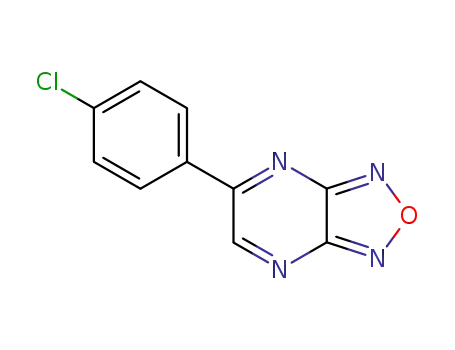
5-(4-chloro-phenyl)-[1,2,5]oxadiazolo[3,4-b]pyrazine
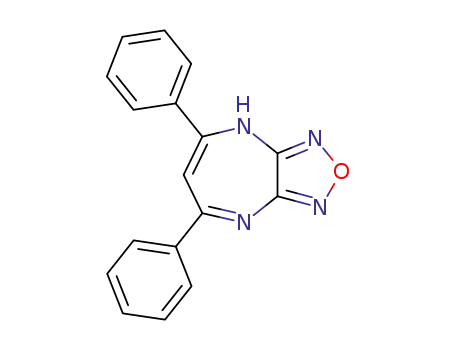
5,7-diphenyl-4H-[1,2,5]oxadiazolo[3,4-b][1,4]diazepine
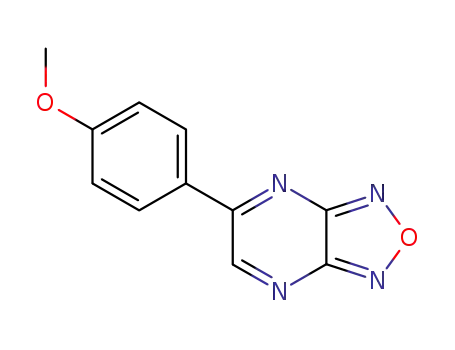
5-(4-methoxyphenyl)furazano[3,4-b]pyrazine
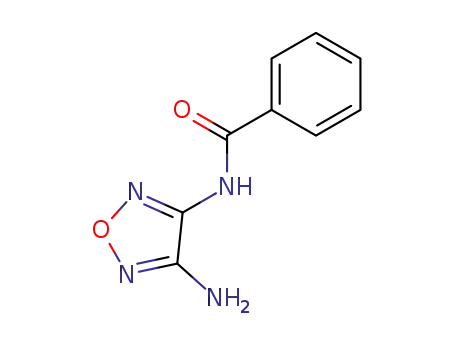
3-amino-4-benzamidofurazan
CAS:112163-33-4
CAS:112-84-5
CAS:114786-39-9
CAS:850140-72-6