- +86-0533-2185556
- WhatsApp: +86 15965530500
- admin@hangyubiotech.com
Your Location:Home >Products >API >119389-05-8
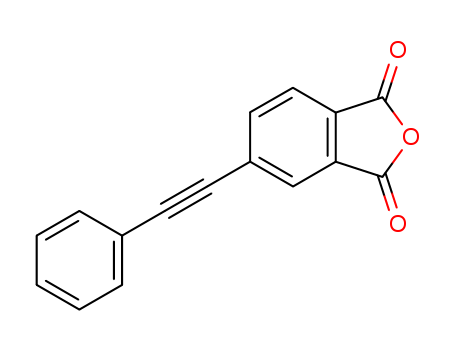
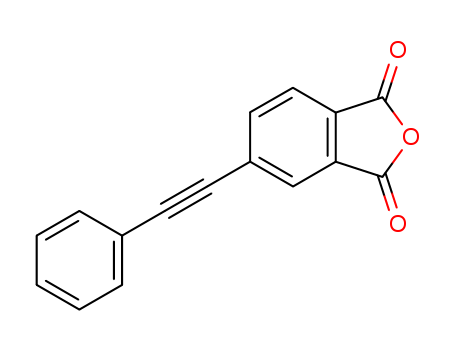
pd_meltingpoint:152 °C
Purity:99%
|
Preparation |
The preparation of 4-phenylethynylphthalic anhydride is as follows:4-phenyl ethynyl dimethyl phthalate (33.6 g) was suspended in a mixed medium comprising water and methanol and a 25% by mass aqueous solution of sodium hydroxide (40 g) was dropwise added thereto with stirring. The resultant reaction mixture was stirred at 60° C. for 3 hours and, then, after confirming the completion of the reaction, cooled to the inside temperature of 30° C. Thereafter, 1 g of active carbon was added thereto, and, then, stirred for 30 minutes maintaining the same temperature. The resultant mixture was filtered to remove the active carbon, and rinsed with water. The filtrate and a rinsing solution were combined and, then, toluene and ethyl acetate were added to the resultant mixture. Concentrated hydrochloric acid (28 g) was dropwise added to the resultant 2-layered reaction mixture. The mixture was stirred for 30 minutes at room temperature and was allowed to stand, so that an organic layer containing 4-phenyl ethynyl phthalic acid was separated. After partially concentrating the organic layer, acetic anhydride (17 g) was added thereto, and the reaction mixture was refluxed with heating for 4 hours. After the reaction was completed, the resultant reaction mixture was cooled, so that 4-phenyl ethynyl phthalic anhydride was precipitated as a crystal. The crystal was filtered, rinsed and dried, to thereby obtain 26.6 g of 4-phenyl ethynyl phthalic anhydride as a pale yellow crystal. The yield was 94% on the basis of 4-phenyl ethynyl dimethyl phthalate. |
InChI:InChI=1/C16H8O3/c17-15-13-9-8-12(10-14(13)16(18)19-15)7-6-11-4-2-1-3-5-11/h1-5,8-10H
Novel magnetic Fe3O4/SiO2/P(GMA-co-EGDMA...
The invention provides an aryl ethynyl p...
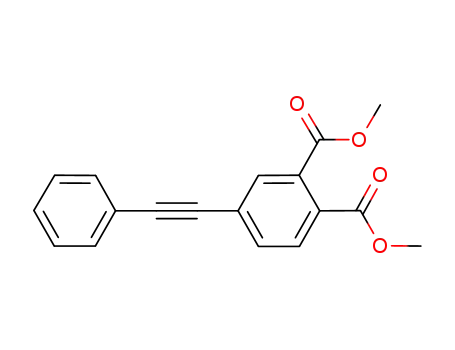
4-phenyl ethynyl dimethyl phthalate

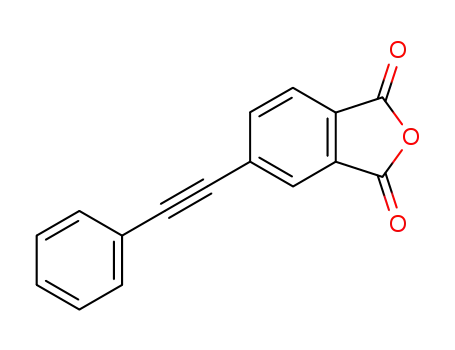
4-(2-phenylethynyl)phthalic anhydride
| Conditions | Yield |
|---|---|
|
4-phenyl ethynyl dimethyl phthalate; With methanol; sodium hydroxide; water; at 60 ℃; for 3h;
With hydrogenchloride; In methanol; diethyl ether; water; at 20 ℃; for 0.5h;
With acetic anhydride; for 4h; Product distribution / selectivity; Heating / reflux;
|
94% |
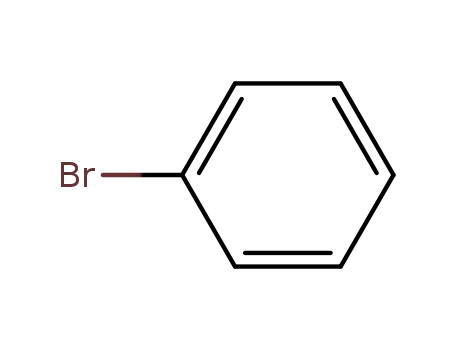
bromobenzene

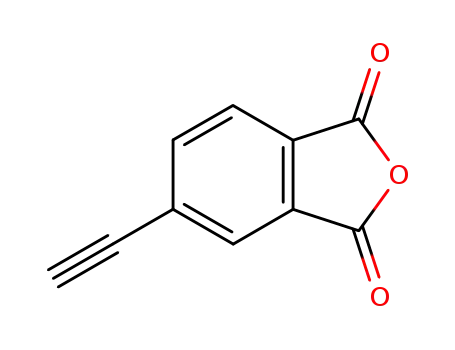
5-ethynylisobenzofuran-1,3-dione

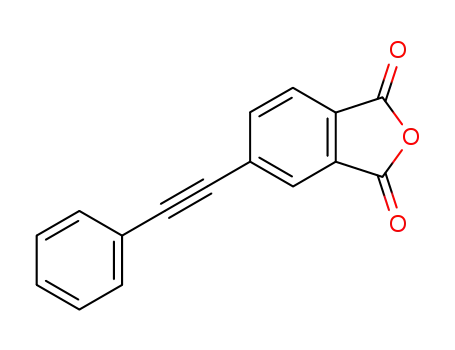
4-(2-phenylethynyl)phthalic anhydride
| Conditions | Yield |
|---|---|
|
With potassium carbonate; In N,N-dimethyl-formamide; at 120 ℃; for 5h;
|
95% |
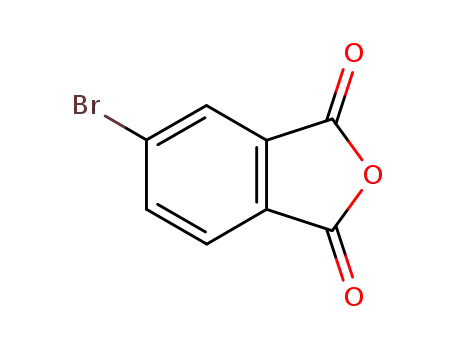
4-bromophthalic anhydride
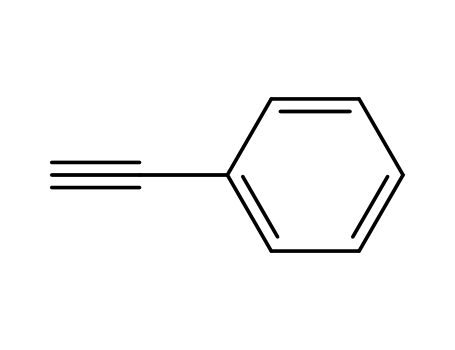
phenylacetylene

4-phenyl ethynyl dimethyl phthalate
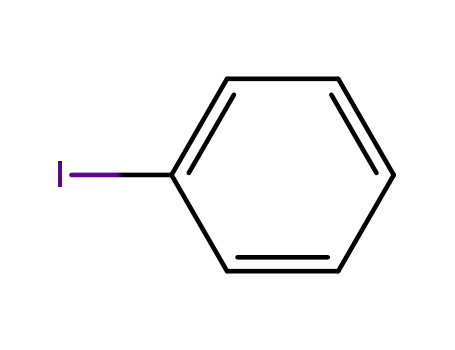
iodobenzene
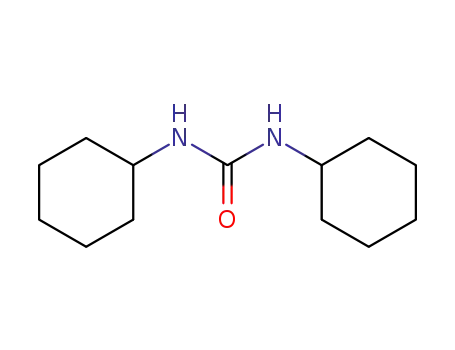
1,3-Dicyclohexylurea
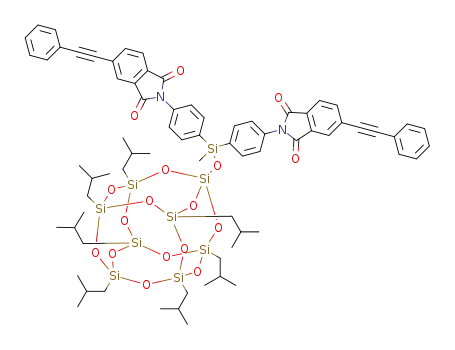
C73H90N2O17Si9
CAS:112163-33-4
CAS:112-34-5
CAS:207739-72-8
CAS:52190-28-0