- +86-0533-2185556
- WhatsApp: +86 15965530500
- admin@hangyubiotech.com
Your Location:Home >Products >small-molecule inhibitor >1350653-24-5
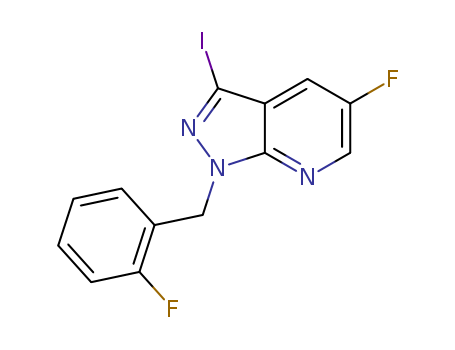

Purity:99%
The present invention relates to a pyraz...
The present application relates to novel...
The present application relates to novel...
The present application relates to novel...
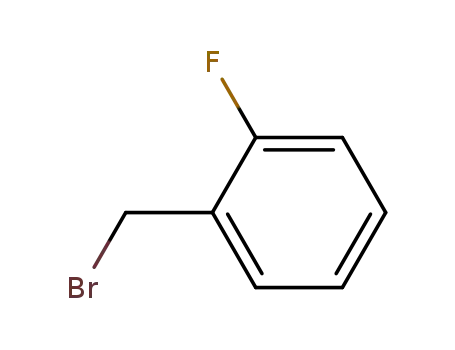
o-fluorobenzyl bromide

![5-fluoro-3-iodo-1H-pyrazolo[3,4-b]pyridine](/upload/2025/4/31a0cd7b-48c0-4980-a06b-037d9184fa33.png)
5-fluoro-3-iodo-1H-pyrazolo[3,4-b]pyridine

![5-fluoro-1-(2-fluorobenzyl)-3-iodo-1H-pyrazolo[3,4-b]pyridine](/upload/2025/4/5af1e1b4-9ae6-48be-98b1-6efd1126907a.png)
5-fluoro-1-(2-fluorobenzyl)-3-iodo-1H-pyrazolo[3,4-b]pyridine
| Conditions | Yield |
|---|---|
|
With
caesium carbonate;
In
N,N-dimethyl-formamide;
at 20 ℃;
for 2h;
|
61% |
|
With
caesium carbonate;
In
N,N-dimethyl-formamide;
at 20 ℃;
for 2h;
|
61% |
|
With
caesium carbonate;
In
N,N-dimethyl-formamide;
at 20 ℃;
for 2h;
|
|
|
With
caesium carbonate;
In
N,N-dimethyl-formamide;
at 20 ℃;
for 2h;
|
|
|
With
caesium carbonate;
In
N,N-dimethyl-formamide;
at 20 ℃;
for 2h;
|
106.6 g |
|
With
caesium carbonate;
In
N,N-dimethyl-formamide;
at 20 ℃;
for 2h;
|
9.0 g |
|
With
caesium carbonate;
In
N,N-dimethyl-formamide;
at 20 ℃;
for 2h;
|
106.6 g |
|
With
caesium carbonate;
In
N,N-dimethyl-formamide;
at 20 ℃;
for 2h;
|
|
|
With
barium carbonate;
In
N,N-dimethyl-formamide;
at 20 ℃;
for 2h;
|
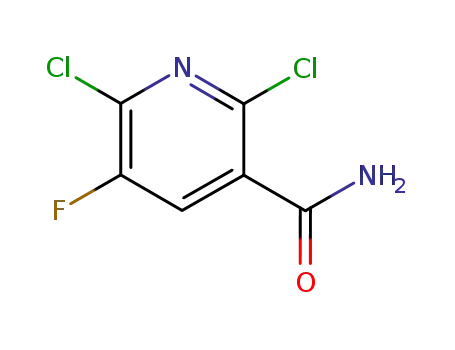
2,6-dichloro-5-fluoropyridine-3-carboxamide

![5-fluoro-1-(2-fluorobenzyl)-3-iodo-1H-pyrazolo[3,4-b]pyridine](/upload/2025/4/5af1e1b4-9ae6-48be-98b1-6efd1126907a.png)
5-fluoro-1-(2-fluorobenzyl)-3-iodo-1H-pyrazolo[3,4-b]pyridine
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 5 steps
1.1: zinc; acetic acid / methanol / 24 h / Reflux
2.1: triethylamine; trifluoroacetic anhydride / dichloromethane / 1.5 h / 0 °C
3.1: hydrazine hydrate / ethylene glycol / 4 h / Reflux
4.1: boron trifluoride diethyl etherate / tetrahydrofuran / 0 °C
4.2: 0.5 h / -10 °C
4.3: 0 - 20 °C
5.1: caesium carbonate / N,N-dimethyl-formamide / 2 h / 20 °C
With
boron trifluoride diethyl etherate; caesium carbonate; hydrazine hydrate; acetic acid; triethylamine; trifluoroacetic anhydride; zinc;
In
tetrahydrofuran; methanol; dichloromethane; ethylene glycol; N,N-dimethyl-formamide;
|
|
|
Multi-step reaction with 5 steps
1.1: zinc; acetic acid / methanol / 24 h / Reflux
2.1: triethylamine; trifluoroacetic anhydride / dichloromethane / 1.5 h / 0 °C
3.1: hydrazine hydrate / ethylene glycol / 4 h / Reflux
4.1: boron trifluoride diethyl etherate; isopentyl nitrite / tetrahydrofuran / -10 - 0 °C
4.2: 0.5 h / 20 °C
5.1: caesium carbonate / N,N-dimethyl-formamide / 2 h / 20 °C
With
boron trifluoride diethyl etherate; caesium carbonate; hydrazine hydrate; acetic acid; triethylamine; trifluoroacetic anhydride; zinc; isopentyl nitrite;
In
tetrahydrofuran; methanol; dichloromethane; ethylene glycol; N,N-dimethyl-formamide;
|
|
|
Multi-step reaction with 5 steps
1.1: zinc; acetic acid / methanol / 24 h / Reflux
2.1: triethylamine; trifluoroacetic anhydride / dichloromethane / 1.5 h / 0 °C
3.1: hydrazine hydrate / ethylene glycol / 4 h / Reflux
4.1: boron trifluoride diethyl etherate; isopentyl nitrite / tetrahydrofuran / 0.5 h / -10 - 0 °C
4.2: 0.5 h / 0 - 20 °C
5.1: caesium carbonate / N,N-dimethyl-formamide / 2 h / 20 °C
With
hydrazine hydrate; boron trifluoride diethyl etherate; caesium carbonate; acetic acid; triethylamine; trifluoroacetic anhydride; zinc; isopentyl nitrite;
In
tetrahydrofuran; methanol; dichloromethane; ethylene glycol; N,N-dimethyl-formamide;
|
|
|
Multi-step reaction with 5 steps
1.1: zinc; acetic acid / methanol / 24 h / Reflux
2.1: trifluoroacetic anhydride; triethylamine / dichloromethane / 1.5 h / 0 °C
3.1: hydrazine hydrate / ethylene glycol / 4 h / Reflux
4.1: boron trifluoride diethyl etherate / tetrahydrofuran / 0 °C
4.2: 0.5 h / -10 °C
4.3: 0.5 h / 0 - 20 °C
5.1: caesium carbonate / N,N-dimethyl-formamide / 2 h / 20 °C
With
boron trifluoride diethyl etherate; caesium carbonate; hydrazine hydrate; acetic acid; triethylamine; trifluoroacetic anhydride; zinc;
In
tetrahydrofuran; methanol; dichloromethane; ethylene glycol; N,N-dimethyl-formamide;
|
|
|
Multi-step reaction with 5 steps
1.1: zinc; acetic acid / methanol / 24 h / Reflux
2.1: triethylamine; trifluoroacetic anhydride / dichloromethane / 1.5 h / 0 °C
3.1: hydrazine hydrate / ethylene glycol / 4 h / Reflux
4.1: boron trifluoride diethyl etherate; isopentyl nitrite / tetrahydrofuran / 0.5 h / -10 - 0 °C
4.2: 0.5 h / 0 - 20 °C
5.1: caesium carbonate / N,N-dimethyl-formamide / 2 h / 20 °C
With
boron trifluoride diethyl etherate; caesium carbonate; hydrazine hydrate; acetic acid; triethylamine; trifluoroacetic anhydride; zinc; isopentyl nitrite;
In
tetrahydrofuran; methanol; dichloromethane; ethylene glycol; N,N-dimethyl-formamide;
|
|
|
Multi-step reaction with 5 steps
1.1: zinc; acetic acid / methanol / 24 h / 20 °C / Reflux
2.1: triethylamine; trifluoroacetic anhydride / dichloromethane / 1.5 h / 0 °C
3.1: hydrazine hydrate / ethylene glycol / 4 h / Reflux
4.1: boron trifluoride diethyl etherate; isopentyl nitrite / tetrahydrofuran / 0.5 h / -10 - 0 °C
4.2: 0.5 h / 0 - 20 °C
5.1: caesium carbonate / N,N-dimethyl-formamide / 2 h / 20 °C
With
boron trifluoride diethyl etherate; caesium carbonate; hydrazine hydrate; acetic acid; triethylamine; trifluoroacetic anhydride; zinc; isopentyl nitrite;
In
tetrahydrofuran; methanol; dichloromethane; ethylene glycol; N,N-dimethyl-formamide;
|
|
|
Multi-step reaction with 5 steps
1.1: zinc; acetic acid / dichloromethane; methanol / 24 h / Reflux
2.1: triethylamine; trifluoroacetic anhydride / dichloromethane / 1.5 h / 0 °C / Reflux
3.1: hydrazine hydrate / ethylene glycol / 4 h / Reflux
4.1: boron trifluoride diethyl etherate; isopentyl nitrite / tetrahydrofuran / 0.5 h / -10 - 0 °C
4.2: 0.5 h / 0 - 20 °C
5.1: caesium carbonate / N,N-dimethyl-formamide / 2 h / 20 °C
With
boron trifluoride diethyl etherate; caesium carbonate; hydrazine hydrate; acetic acid; triethylamine; trifluoroacetic anhydride; zinc; isopentyl nitrite;
In
tetrahydrofuran; methanol; dichloromethane; ethylene glycol; N,N-dimethyl-formamide;
|
|
|
Multi-step reaction with 5 steps
1.1: zinc; acetic acid / methanol / 24 h / 20 °C / Reflux
2.1: triethylamine; trifluoroacetic anhydride / dichloromethane / 1.5 h / 0 °C
3.1: hydrazine hydrate / ethylene glycol / 4 h / Reflux
4.1: boron trifluoride diethyl etherate; isopentyl nitrite / tetrahydrofuran / 0.5 h / -10 - 0 °C
4.2: 0.5 h / 0 - 20 °C
5.1: caesium carbonate / N,N-dimethyl-formamide / 2 h / 20 °C
With
boron trifluoride diethyl etherate; caesium carbonate; hydrazine hydrate; acetic acid; triethylamine; trifluoroacetic anhydride; zinc; isopentyl nitrite;
In
tetrahydrofuran; methanol; dichloromethane; ethylene glycol; N,N-dimethyl-formamide;
|
|
|
Multi-step reaction with 5 steps
1.1: zinc; acetic acid / methanol / 24 h / 80 °C
2.1: trichlorophosphate / chloroform / 8 h / 80 °C
3.1: hydrazine hydrate / 1,2-dimethoxyethane / 180 °C
4.1: boron trifluoride diethyl etherate; isopentyl nitrite / tetrahydrofuran / 0.5 h / -10 °C
4.2: 0.5 h / 0 - 20 °C
5.1: barium carbonate / N,N-dimethyl-formamide / 2 h / 20 °C
With
hydrazine hydrate; boron trifluoride diethyl etherate; acetic acid; barium carbonate; zinc; trichlorophosphate; isopentyl nitrite;
In
tetrahydrofuran; methanol; 1,2-dimethoxyethane; chloroform; N,N-dimethyl-formamide;
|

o-fluorobenzyl bromide
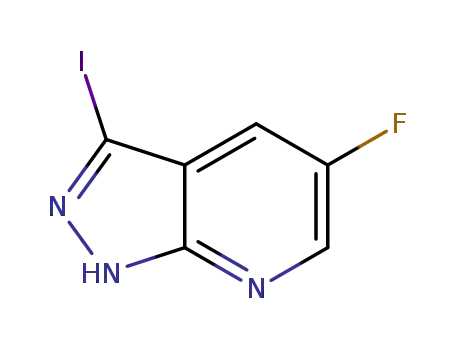
5-fluoro-3-iodo-1H-pyrazolo[3,4-b]pyridine

2,6-dichloro-5-fluoropyridine-3-carboxamide
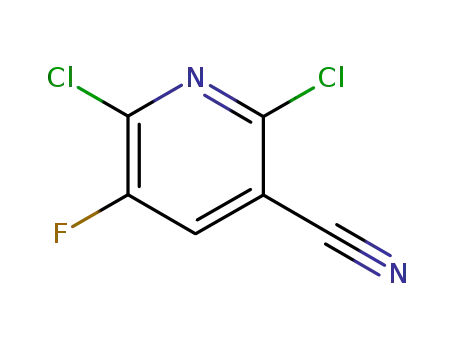
2,6-dichloro-5-fluoro-pyridine-3-carbonitrile
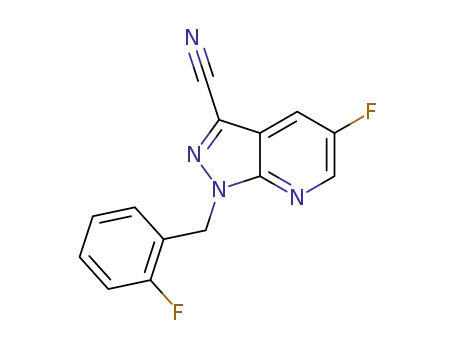
5-fluoro-1-(2-fluorobenzyl)-1H-pyrazolo[3,4-b]pyridine-3-carbonitrile
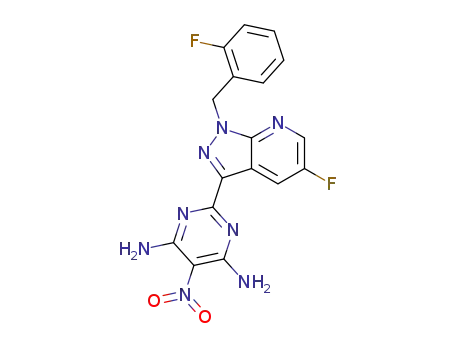
2-[5-fluoro-1-(2-fluorobenzyl)-1H-pyrazolo[3,4-b]pyridin-3-yl]-5-nitropyrimidine-4,6-diamine
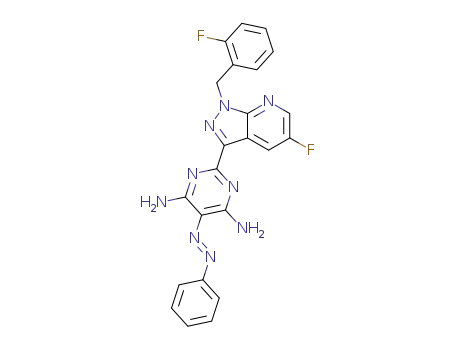
2-[5-fluoro-1-(2-fluorobenzyl)-1H-pyrazolo[3,4-b]pyridin-3-yl]-5-[(E)-phenyldiazenyl]pyrimidine-4,6-diamine
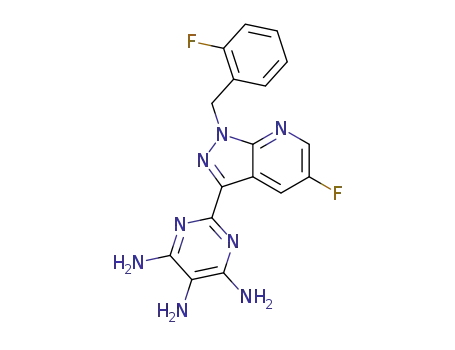
2-[5-fluoro-1-(2-fluorobenzyl)-1H-pyrazolo[3,4-b]pyridin-3-yl]pyrimidine-4,5,6-triamine
CAS:1310726-60-3
CAS:109-15-9
CAS:552301-45-8
CAS:187235-37-6