- +86-0533-2185556
- WhatsApp: +86 15965530500
- admin@hangyubiotech.com
Your Location:Home >Products >small-molecule inhibitor >481-53-8
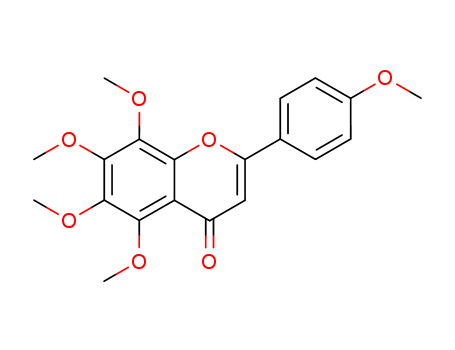

pd_meltingpoint:153.0 to 157.0 °C
Appearance:yellow crystalline
Purity:99%
|
Definition |
ChEBI: A pentamethoxyflavone flavone with methoxy groups at positions 4', 5, 6 , 7 and 8. |
InChI:InChI=1/C20H20O7/c1-22-12-8-6-11(7-9-12)14-10-13(21)15-16(23-2)18(24-3)20(26-5)19(25-4)17(15)27-14/h6-10H,1-5H3
Abstract: A series of polymethoxyflavono...
Disclosed is a use of flavones derivativ...
Disclosed is a use of flavones derivativ...
Fractionation of the bioactive dichlorom...
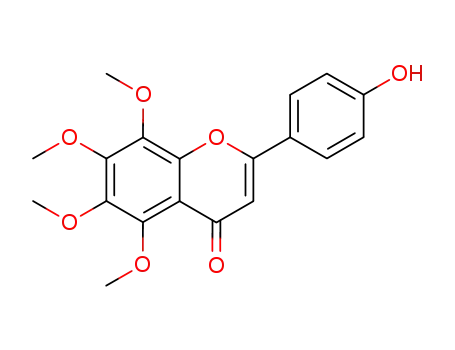
4'-hydroxy-5,6,7,8-tetramethoxy flavone

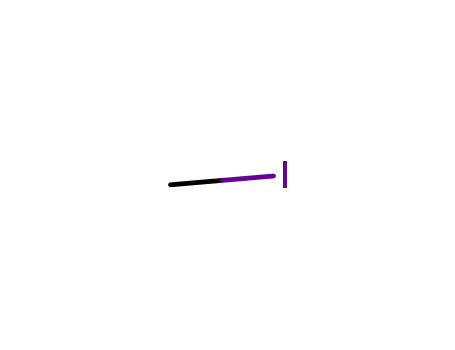
methyl iodide

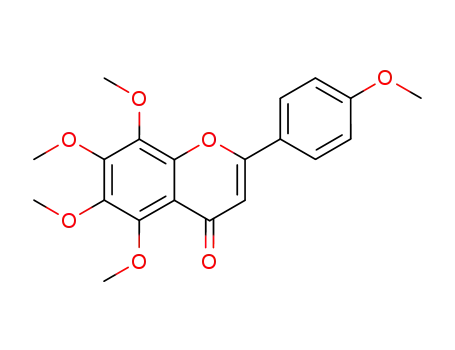
tangeritin
| Conditions | Yield |
|---|---|
|
With
potassium carbonate;
In
N,N-dimethyl-formamide;
at 20 ℃;
for 5h;
Inert atmosphere;
|
84% |
|
With
potassium carbonate;
In
acetone;
for 5h;
Heating;
|
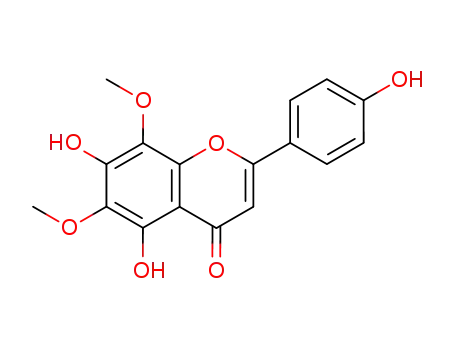
5,7-dihydroxy-2-(4-hydroxyphenyl)-6,8-dimethoxy-4H-chromen-4-one

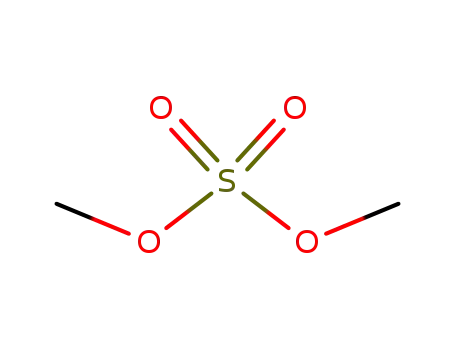
dimethyl sulfate


tangeritin
| Conditions | Yield |
|---|---|
|
With
potassium carbonate;
In
acetone;
at 65 ℃;
for 5h;
|
86% |
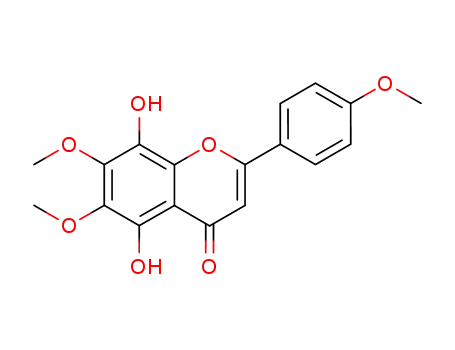
5,8-dihydroxy-6,7-dimethoxy-2-(4-methoxyphenyl)-4-benzopyrone

dimethyl sulfate
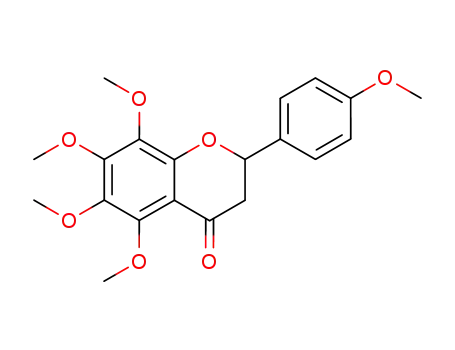
5,6,7,8,4'-penta methoxyl-flavanone
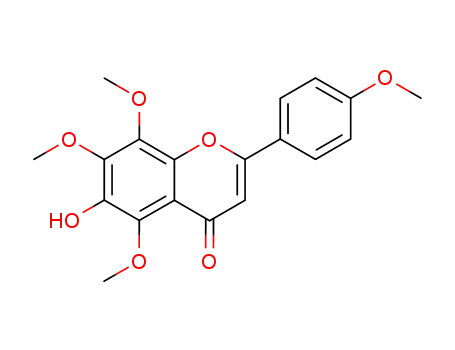
6-hydroxy-4',5,7,8-tetramethoxyflavone
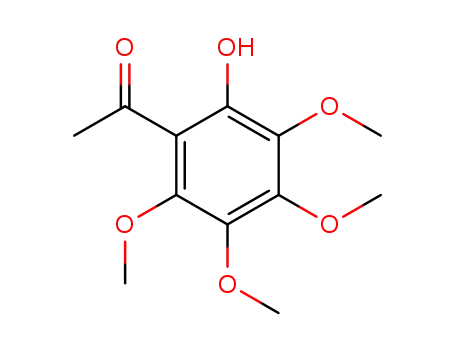
2-hydroxy-3,4,5,6-tetramethoxyacetophenone
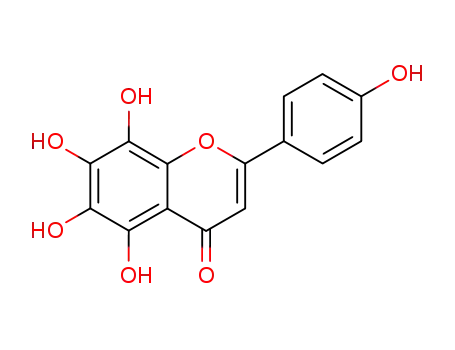
NSC76988
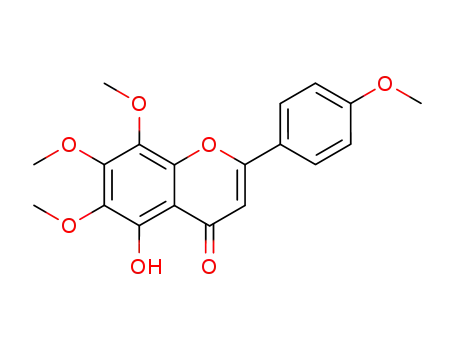
gardenin B
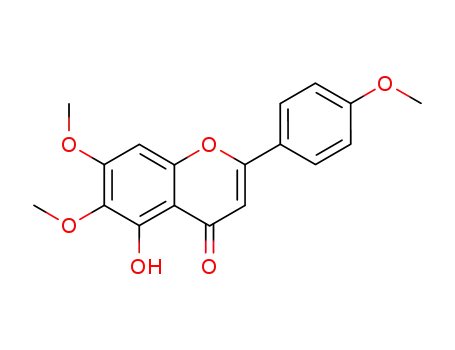
5-hydroxy-4',6,7-trimethoxyflavone
CAS:112163-33-4
CAS:112-34-5
CAS:10296-76-1