- +86-0533-2185556
- WhatsApp: +86 15965530500
- admin@hangyubiotech.com
Your Location:Home >Products >API >104347-13-9
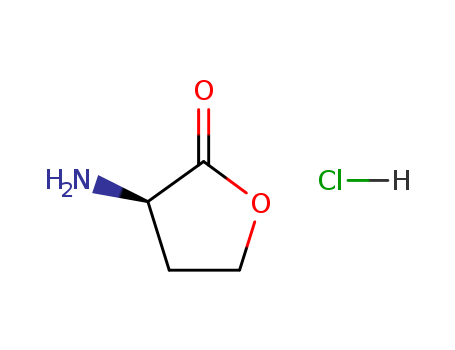

pd_meltingpoint:220-224 °C
Purity:99%
InChI:InChI=1/C4H7NO2.ClH/c5-3-1-2-7-4(3)6;/h3H,1-2,5H2;1H/t3-;/m1./s1
(RS)-α-Amino-γ-butyrolactone hydrochlori...
The invention technical field of chemica...
The invention discloses a preparation me...
The enzymatic resolution of the N-phenyl...
A new stereocontrolled approach to the s...
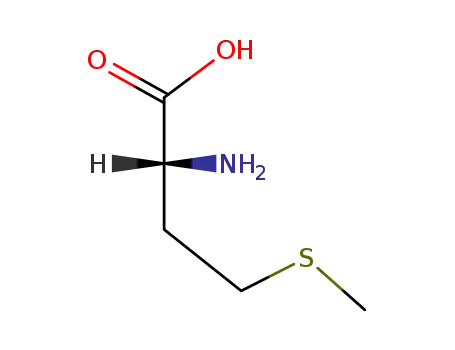
D-methionine

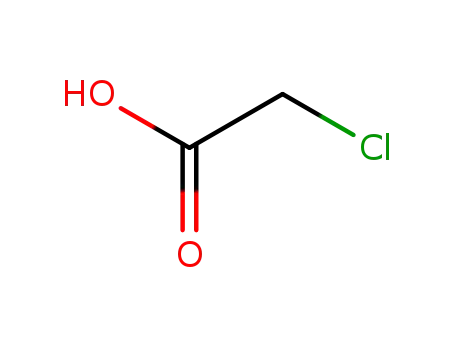
chloroacetic acid


methylsulfanyl-acetic acid

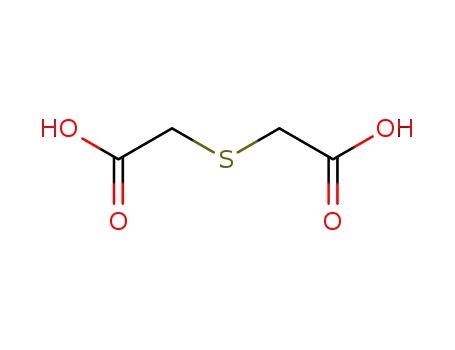
thiodiacetic acid

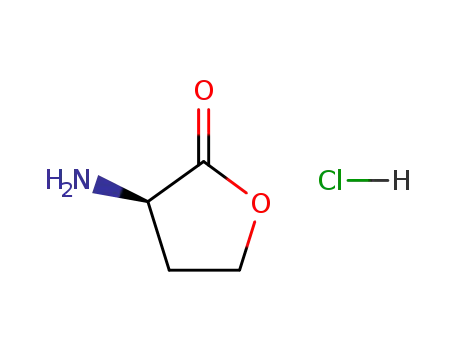
(R)-(+)-α-amino-γ-butyrolactone hydrochloride
| Conditions | Yield |
|---|---|
|
In water; at 80 ℃; for 8h; Overall yield = 268.4 g;
|
α-Phenylacetamido-γ-Butyrolactone

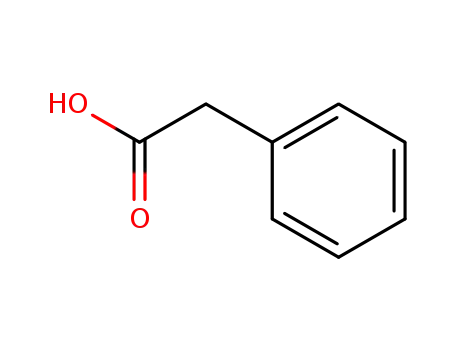
phenylacetic acid


(R)-(+)-α-amino-γ-butyrolactone hydrochloride

(S)-N-(2-oxo-tetrahydrofuran-3-yl)-2-phenylacetamide
| Conditions | Yield |
|---|---|
|
α-Phenylacetamido-γ-Butyrolactone; With ammonia; water; at 25 ℃; for 1h; pH=7.5; Enzymatic reaction;
With hydrogenchloride; In water; pH=2; enantioselective reaction;
|
47% 46% |
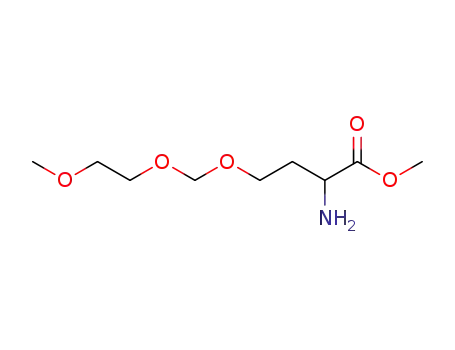
2-Amino-4-(2-methoxy-ethoxymethoxy)-butyric acid methyl ester
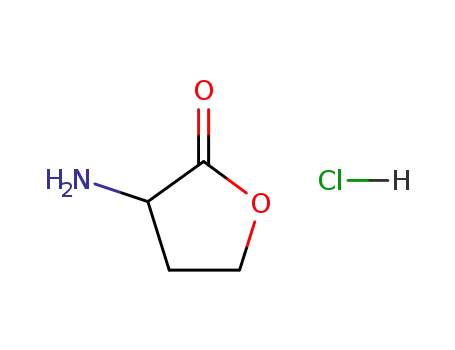
(RS)-α-amino-γ-butyrolactone hydrochloride

D-methionine
α-Phenylacetamido-γ-Butyrolactone
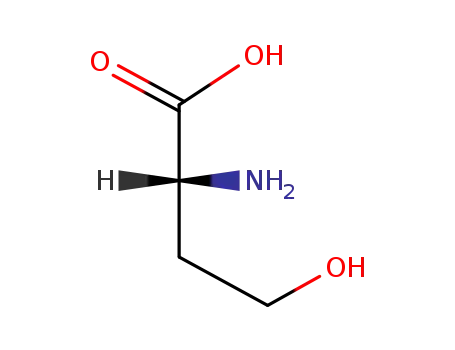
D-homoserine
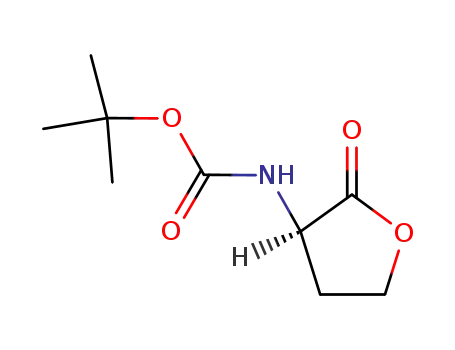
(R)-2-[(tert-butoxycarbonyl)amino]butano-4-lactone
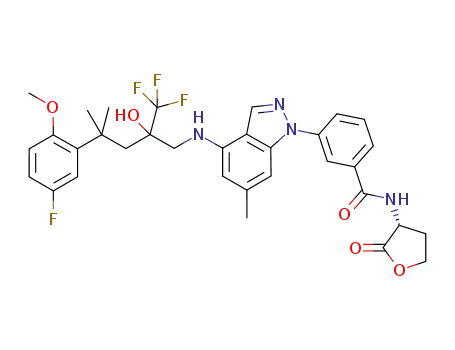
3-(4-{[4-[5-fluoro-2-(methyloxy)phenyl]-2-hydroxy-4-methyl-2-(trifluoromethyl)pentyl]amino}-6-methyl-1H-indazol-1-yl)-N-[(3R)-2-oxotetrahydro-3-furanyl]benzamide
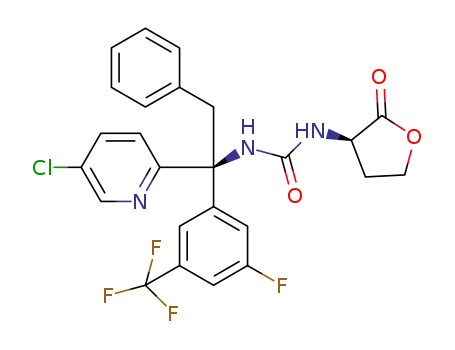
C25H20ClF4N3O3
CAS:118685-33-9
CAS:6138-41-6
CAS:32959-62-9
CAS:104054-27-5