- +86-0533-2185556
- WhatsApp: +86 15965530500
- admin@hangyubiotech.com
Your Location:Home >Products >small-molecule inhibitor >104054-27-5
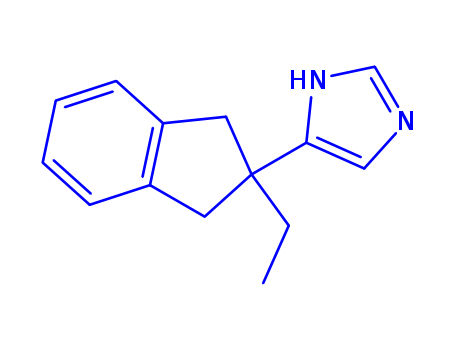

Appearance:Off-white to light yellow crystal powder
Purity:99%
|
Manufacturing Process |
(a). 2-Acetyl-1-indanone (Liebigs Ann. Chem. 1906, 347, 112) is alkylated with ethylbromide in acetone in the presence of sodium carbonate to 2-acetyl- 2-ethyl-1-indanone. The acetyl group is brominated with bromine in methanol and to imidazole by heating in formamide as before. The melting point of the 4(5)-(2,3-dihydro-2-ethyl-1-oxo-1H-inden-2-yl)imidazole is 126-127°C (from ethyl acetate). (b). The carbonyl group of 4(5)-(2,3-dihydro-2-ethyl-1-oxo-1H-inden-2- yl)imidazole is reduced to the alcohol group with sodium borohydride in ethanol. The product is the mixture of cis-trans stereoisomers, the purification of which is accomplished by liquid chromatography: cis-isomer as hydrochloride (melting point 184-185°C), 1H NMR (80 MHz, MeOH-d4): 0.73 (3H, t), 1.86 (2H, m), 3.36 (2H, m), 3.61 (3H, s), 5.15 (1H, s), 7.06 (1H, d), 7.2-7.4 (4H, m), 8.69 (1H, d), and trans-isomer as hydrochloride, 1H NMR (80 MHz, MeOH-d4): 0.80 (3H, t), 1.84 (2H, m), 3.15 (2H, m), 3.24 (3H, s), 5.15 (1H, s), 6.87 (1H, d), 7.2-7.4 (4H, m), 8.54 (1H, d). The oxo derivative prepared in step (a) or the hydroxy derivative prepared in step (b) is hydrogenated in 2 N hydrochloric acid in the presence of 10% palladium on carbon at 70°C. When the uptake of hydrogen ceases, the reaction mixture is filtered and made alkaline. The product is extracted with methylene chloride which is washed with water, dried and evaporated to dryness. From the residue, which is the product as base, is made the hydrochloride of 4(5)-(2,3-dihydro-2-ethyl-1H-inden-2-yl)imidazole using dry hydrogen chloride in ethyl acetate. It has melting point 211-215°C. |
|
Therapeutic Function |
Antihypertensive |
|
Biochem/physiol Actions |
Atipamezole is a selective α2 adrenergic blocker. Atipamezole is more potent than yohimbine; it is very selective for α2 adrenergic vs α1 sites, but not selelctive for α2 subtypes. |
|
Brand name |
Antisedan (Farmos Group Ltd., Finland). |
|
General Description |
Atipamezole has an imidazole structure and gets localized in the central nervous system on administration. |
InChI:InChI=1/C14H16N2/c1-2-14(13-9-15-10-16-13)7-11-5-3-4-6-12(11)8-14/h3-6,9-10H,2,7-8H2,1H3,(H,15,16)
The invention relates to the technical f...
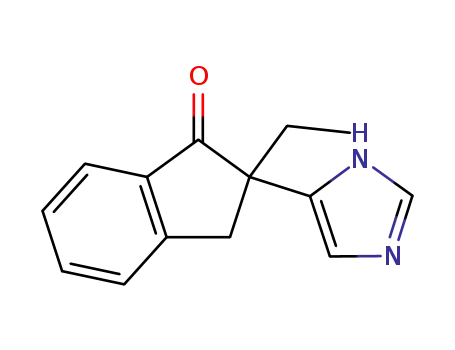
4-(2-ethyl-1-indanone-2-yl)imidazole

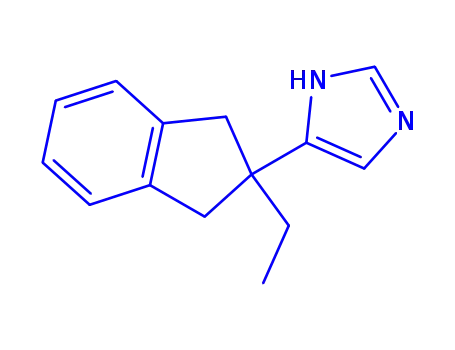
atipamezole
| Conditions | Yield |
|---|---|
|
With
hydrazine hydrate; sodium hydroxide;
In
water; diethylene glycol;
at 175 ℃;
for 7h;
Solvent;
Temperature;
Inert atmosphere;
|
212 g |

atipamezole

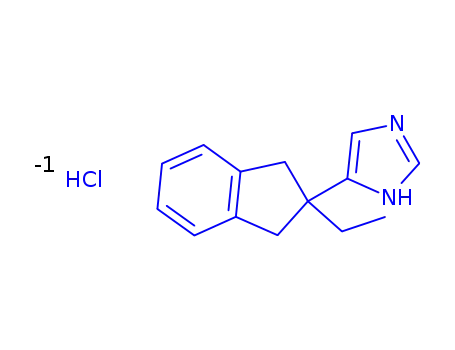
atipamezole hydrochloride
| Conditions | Yield |
|---|---|
|
With
hydrogenchloride;
In
water;
|

atipamezole hydrochloride
CAS:112163-33-4
CAS:112-84-5
CAS:104347-13-9
CAS:446262-90-4