- +86-0533-2185556
- WhatsApp: +86 15965530500
- admin@hangyubiotech.com
Your Location:Home >Products >intermediate >124-20-9

pd_meltingpoint:23-25 °C
Appearance:Colorless clear liquid
Purity:99%
|
Source |
Spermidine is found in fresh green pepper, wheat germ, cauliflower, broccoli, mushrooms, and a variety of cheeses. Even higher amounts are found in soybean products such as natto, shitake mushrooms, amaranth grain and durian. Some of these ingredients are particularly common in a Mediterranean diet, which may explain why some believe eating like the Spanish can prolong your life. |
|
Preparation |
The linker resin 2 was used in the synthesis of spermidine. In order to initiate the preparation the 2-nitrossulfonamide was anchored on the resin 2 by reaction in THF under reflux. The resulting resin 3 then reacted with 4-bromobutylphthalimide in acetonitrile in the presence of Cs2CO3 as base, generating the resin 4. Protected spermidine 5 was cleaved from resin 4 after treatment with hydrate hydrazine at room temperature. Spermidine 5 was previously prepared by our group in solution system utilizing Fukuyama's sulfonamide.Synthesis and Characterization of a Linker for Primary Amines used in the Solid Phase Organic Synthesis of SpermidineJ. Braz. Chem. Soc., Vol. 22, No. 1, 86-91, 2011DOI: 10.1590/S0103-50532011000100011 |
|
Mode of action |
Spermidine's main mechanism of action is its ability to induce autophagy, a self-preservation process which clears out toxic, damaged and dysfunctional cells, resulting in lower levels of inflammation in the body. Autophagy is the main mechanism of spermidine in delaying aging and prolonging the lifespan. In addition, spermidine exerts its effects through other mechanisms, including anti-inflammation, histone acetylation reduction, lipid metabolism and regulation of cell growth and signaling pathways. |
|
General Description |
Spermidine is a naturally occurring polyamine involved in cellular growth regulation and protein synthesis, with potential implications in cancer therapy due to its role in modulating ornithine decarboxylase (ODC) activity and influencing neoplastic cell proliferation. Its analogs and derivatives have been explored for their antineoplastic properties, particularly in targeting malignancies such as melanoma and carcinoma. Additionally, spermidine's interaction with ODC highlights its significance in polyamine metabolism, making it a candidate for therapeutic interventions aimed at inhibiting aberrant cellular growth. |
|
Application |
Spermidine serves as a precursor of spermine and essential for both normal and neoplastic tissue growth. It was first detected in human sperm, but occurs widely in nature. It is essential in both normal and neoplastic tissue growth. Spermidine has a role in cell growth processes and the formation and interconversion of spermidine in mammalian cells has been reported. It has been studied in the regulation of tRNA methyltransferase activity and stimulates T4 polynucleotide kinase activity. |
|
Definition |
ChEBI: Spermidine is a triamine that is the 1,5,10-triaza derivative of decane. It is a polyamine that is routinely included in restriction enzyme digestions to improve the cleavage efficacy of the DNA. Spermidine counteracts the inhibitory effects of contaminants coisolated with DNA and consequently permits complete digestion of the DNA at lower enzyme concentrations. |
InChI:InChI=1/C7H19N3/c8-4-1-2-6-10-7-3-5-9/h10H,1-9H2/p+3
Spermine oxidase (SMO) and acetylpolyami...
Flavoprotein Fms1 from Saccharomyces cer...
In mammalian cells, the flavoprotein spe...
Lysine 315 of mouse polyamine amine oxid...
-
The invention relates to the technical f...
The invention provides a synthesis proce...
The invention belongs to the field of ch...
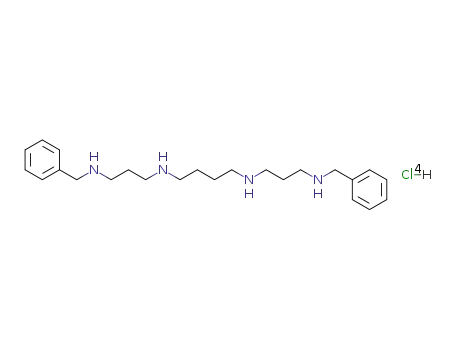
1,14-bis(phenylmethyl)-1,5,10,14-tetraazatetradecane tetrahydrochloride

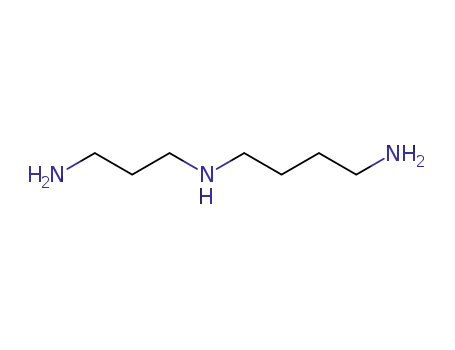
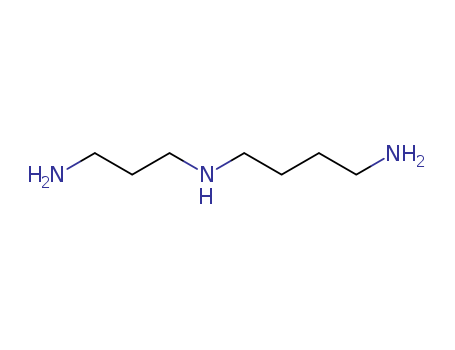
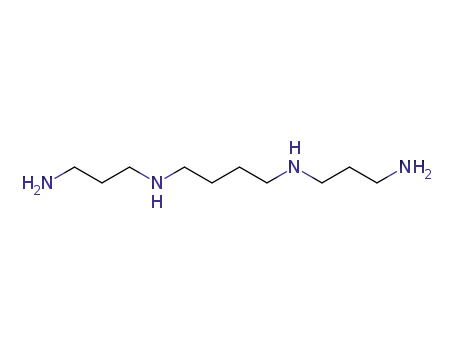
N-(3-aminopropyl)-1,4-diaminobutane

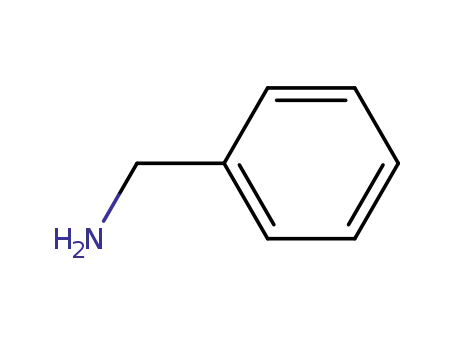
benzylamine
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 2 steps
1: recombinant human polyamine oxidase / pH 9.5 / Enzymatic reaction
2: spermine oxidase / pH 9.5 / Enzymatic reaction
With
recombinant human polyamine oxidase; spermine oxidase;
|

1,14-bis(phenylmethyl)-1,5,10,14-tetraazatetradecane tetrahydrochloride


N-(3-aminopropyl)-1,4-diaminobutane

Spermine

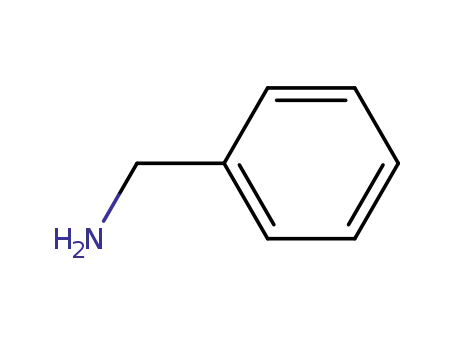
benzylamine
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 2 steps
1: recombinant human polyamine oxidase / pH 9.5 / Enzymatic reaction
2: recombinant human polyamine oxidase / pH 9.5 / Enzymatic reaction
With
recombinant human polyamine oxidase;
|
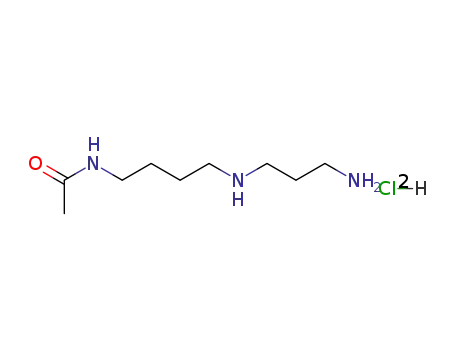
N8-acetylspermidine dihydrochloride
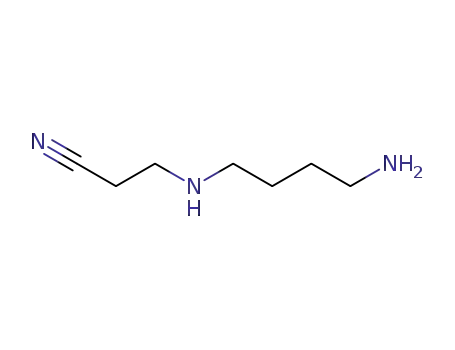
N-(cyanoethyl)-1,4-diaminobutane

Spermine
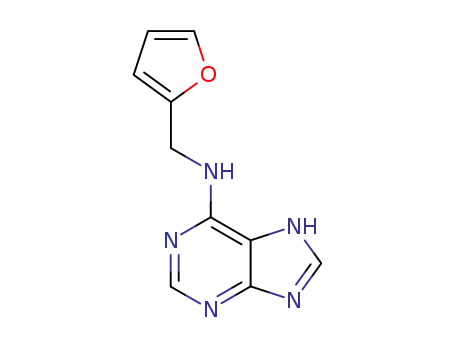
kinetin

N1,N8-dicinnamoyl spermidine
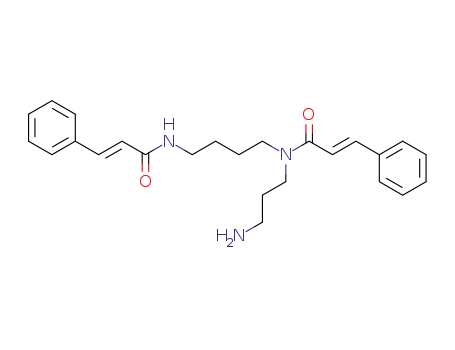
N',N''-Dicannamoylspermidin
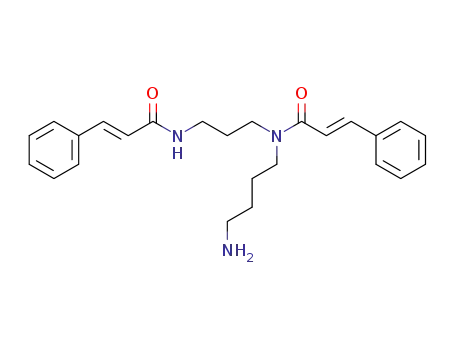
N,N'-Dicannamoylspermidin
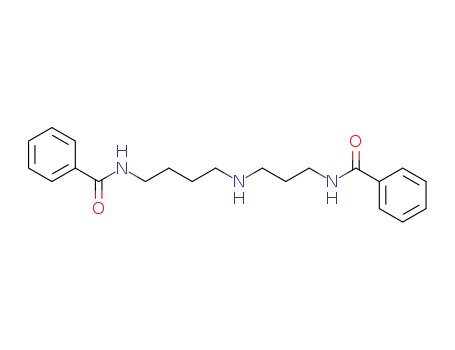
N1,N8-bis(benzoyl)spermidine
CAS:112163-33-4
CAS:112-84-5
CAS:113852-37-2
CAS:1191237-69-0