- +86-0533-2185556
- WhatsApp: +86 15965530500
- admin@hangyubiotech.com
Your Location:Home >Products >small-molecule inhibitor >29296-32-0
pd_meltingpoint:111-114oC(lit.)
Purity:99%
|
General Description |
1,4,10,13-Tetraoxa-7,16-diazacyclooctadecane, also known as cyclam, is a macrocyclic compound that contains four oxygen and two nitrogen atoms in its structure. It is a versatile chemical that is widely used in various fields including coordination chemistry, materials science, and peptide chemistry. Cyclam is known for its ability to form stable complexes with metal ions, making it useful in metal extraction and separation processes. It also has applications in the development of functional materials such as molecular sieves and catalysts. Additionally, cyclam derivatives have been studied for their potential use in drug delivery and medical imaging. Overall, the unique structure and properties of 1,4,10,13-Tetraoxa-7,16-diazacyclooctadecane make it a valuable and versatile chemical in the field of chemistry and materials science. |
InChI:InChI=1/C12H26N2O4/c1-5-15-9-10-17-7-3-14-4-8-18-12-11-16-6-2-13-1/h13-14H,1-12H2/p+2
The invention relates to the field of or...
The invention relates to a synthesis met...
The direct selective halogenation of una...
Molecular structures of the most promine...
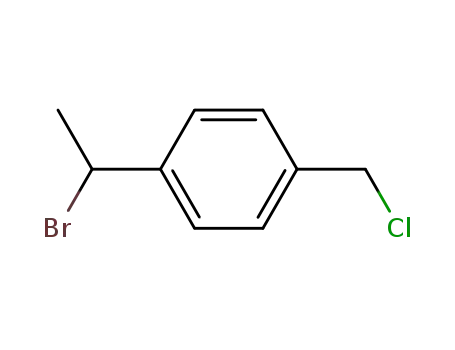
p-chloromethyl-α-bromoethylbenzene

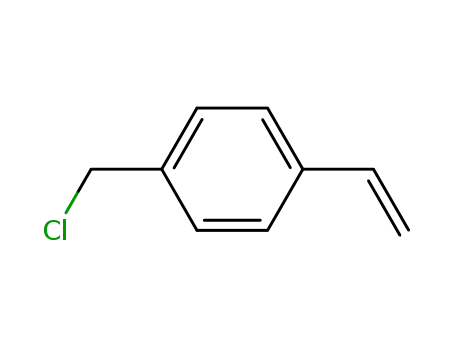
4-Vinylbenzyl chloride
| Conditions | Yield |
|---|---|
|
With
18-crown-6 ether; potassium hydroxide;
In
toluene;
at 40 ℃;
for 4h;
Reagent/catalyst;
Temperature;
Solvent;
|
95% |
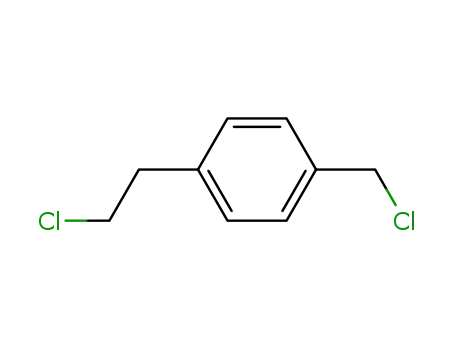
1-(2-chloroethyl)-4-(chloromethyl)benzene

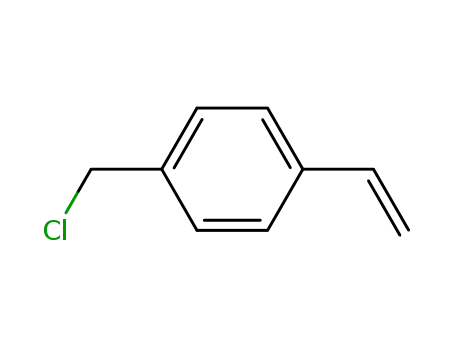
4-Vinylbenzyl chloride
| Conditions | Yield |
|---|---|
|
With
4-tert-Butylcatechol; sodium t-butanolate;
In
tetrahydrofuran;
at 30 ℃;
for 1h;
Reagent/catalyst;
Solvent;
Inert atmosphere;
|
83% |
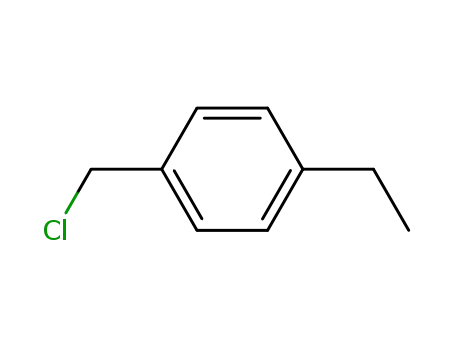
4-ethylbenzylchloride
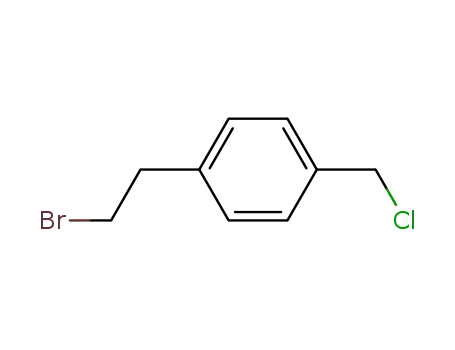
p-chloromethyl-2-bromoethylbenzene
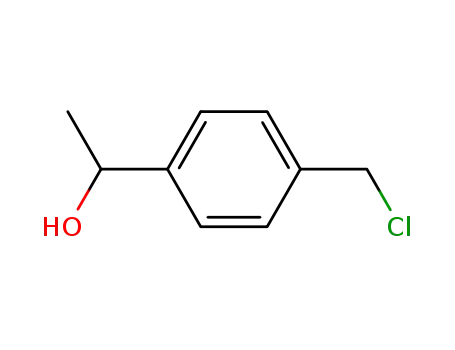
1-(4-chloromethylphenyl)ethanol
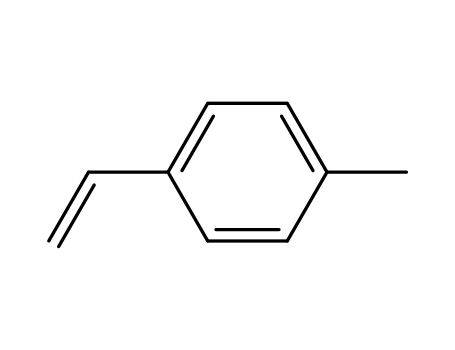
1-ethenyl-4-methylbenzene
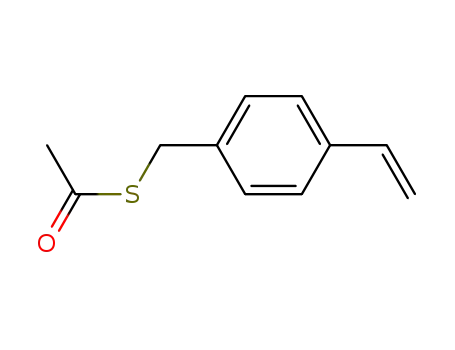
4-vinylbenzyl thioacetate
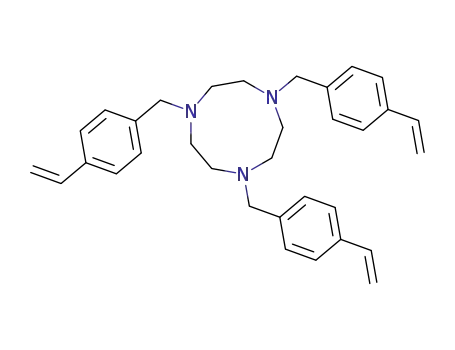
1,4,7-Tris-(4-vinyl-benzyl)-[1,4,7]triazonane
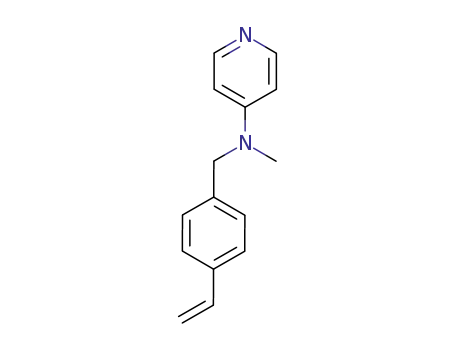
4-[N-methyl-N-(p-vinylbenzyl)amino]pyridine
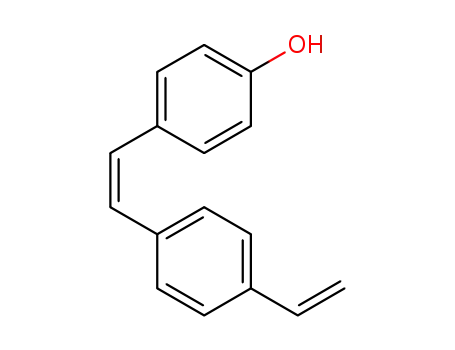
(Z)-4-hydroxy-4'-vinylstilbene
CAS:112163-33-4
CAS:112-84-5
CAS:147116-67-4
CAS:26338-45-4