- +86-0533-2185556
- WhatsApp: +86 15965530500
- admin@hangyubiotech.com
Your Location:Home >Products >Pharmaceutical intermediate >104206-82-8
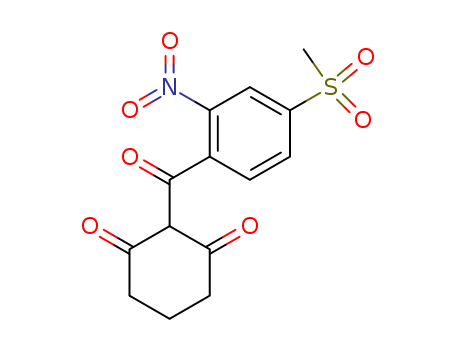

pd_meltingpoint:165oC
Appearance:Light yellow crystalline powder
Purity:99%
|
Chemical and Physical Properties |
Mesotrione is a light yellow opaque solid with a faint pleasant odour. It dissolves at a rate of 2.2 g/l at 200 C in unbuffered water whose pH is 4.8. It also dissolves in n-heptane <0.5, xylene 1.6, toluene 3.1, methanol 4.6, ethyl acetate 18.6, 1,2-dichloroethane 66.3, acetone 93.3, and in acetonitrile 117.0, all in g/l at 200 C. Mesotrione has a molecular weight of 339.318 g/mol, a monoisotopic mass of 339.041 g/mol and an exact mass of 339.041 g/mol. It has a heavy atom count of 23 and a complexity of 627. |
|
Mechanism of Action |
Studies indicate that the toxic influence of the compound can be attributed to high plasma concentration levels of tyrosine which is resultant of inhibition by 4-hydroxyphenylpyruvate dioxygenase (HPPD). HPPD is an essential constituent of the biochemical route that aids the conversion of tyrosine to alpha-tocopherol and plastoquino\ne. Mesotrione is a highly potent agonist of HPPD against Arabidopsis thaliana, which is indicated by the Ki figure of c 6-18 pM. It is readily absorbed by weed species after foliar application and it is widely distributed to various parts of the plant through basipetal and acropetal movement. Maize crops are generally tolerant to Mesotrione as a result of selective metabolism by the plant. The compound may take a relatively long time to be absorbed in some weed species hence Mesotrione is applied as a selective herbicide in maize plantations. The plasma half-life of the compound is approximately 1 hour. |
|
Preparation |
Mesotrione is synthesized through the reaction of acetyl chloride with 3-methylmercaptonitrobenzene with aluminum trichloride as the catalyst. The relative ketone is oxidized with sodium oxychloride which results in the 4-methylsulfonyl compound, which is condensed with cyclohexane-1,3-dione to obtain the respective enol ester. The latter is reorganized to the appropriate form of the product with potassium cyanide with triethylamine as the catalyst.? Mesotrione is available as a soluble solid or concentrates, as a pressurized liquid, a ready to use solution, soluble concentrate, emulsifiable concentrate, water dispersible granules and in granular form.? Some of its premix partners may include terbuthylazine, Rimsulfron, Nicosulfron, S-Metolachlor, Glyphosphate and Atrazine. |
|
Consumption |
A study indicated that the total usage of the compound in the last 5 years is primarily in corn, which accounts for about 98% of the total acres treated and the total pounds of the applied Mesotrione. Field corn accounts for the highest percentage of the treated crops in regards to the acres treated and the acreage of crops grown at 20.2%. |
|
Precautionary Statements |
The applicator should put on protective gear when handling Mesotrione concentrate or contaminated surfaces. It is recommended that one avoids eating, drinking or smoking while handling the product and that one should always wash off any splashes that come into contact with the skin immediately.? During the application process, the sprayer should ensure that the machine is calibrated appropriately, the nozzles are in a matched set and the machine is adjusted to the respective level of the crop. The concentrates should not be left in the sprayer for extended periods of time.? Mesotrione may cause mild or acute eye irritation. However, it is not a dermal irritant. |
|
Definition |
ChEBI: An aromatic ketone that is cyclohexa-1,3-dione in which one of the hydrogens at position 2 is substituted by a 4-(methanesulfonyl)-2-nitrobenzoyl group. |
InChI:InChI=1/C14H13NO7S/c1-23(21,22)8-5-6-9(10(7-8)15(19)20)14(18)13-11(16)3-2-4-12(13)17/h5-7,13H,2-4H2,1H3
The invention provides a preparation met...
The invention relates to a method for pr...
The development of eco-friendly and swit...
The recent development of selective oxid...
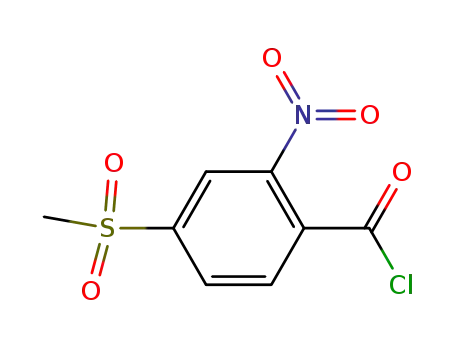
4-methylsulphonyl-2-nitrobenzoyl chloride

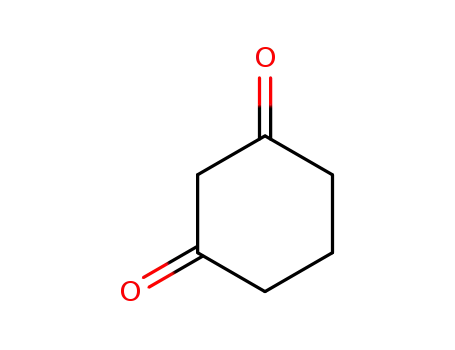
1,3-cylohexanedione

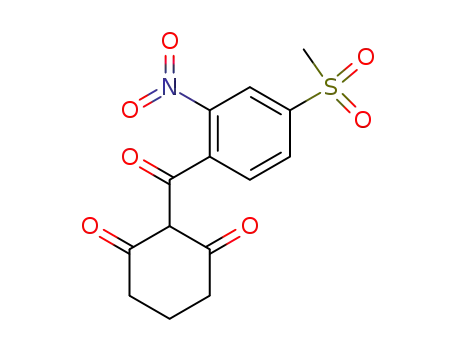
mesotrione
| Conditions | Yield |
|---|---|
|
With
triethylamine; 2-hydroxy-2-methylpropanenitrile;
In
dichloromethane;
at 15 ℃;
Large scale;
|
89.66% |
|
With
triethylamine;
In
dichloromethane;
at 35 - 40 ℃;
|
88.74% |
|
4-methylsulphonyl-2-nitrobenzoyl chloride; 1,3-cylohexanedione;
In
dichloromethane;
at 15 ℃;
for 2h;
With
2-hydroxy-2-methylpropanenitrile;
In
dichloromethane;
at 32 ℃;
for 3h;
Temperature;
|
88% |
|
4-methylsulphonyl-2-nitrobenzoyl chloride; 1,3-cylohexanedione;
With
15-crown-5; sodium hydroxide;
In
chloroform;
at 35 ℃;
for 1.5h;
With
15-crown-5; sodium hydroxide; N,N,N',N'-tetramethylguanidine;
In
chloroform;
at 350 ℃;
for 3.5h;
Solvent;
Temperature;
|
88.98% |
|
4-methylsulphonyl-2-nitrobenzoyl chloride; 1,3-cylohexanedione;
With
triethylamine;
In
1,2-dichloro-ethane;
at -10 - 50 ℃;
for 0.0333333h;
With
2-hydroxy-2-methylpropanenitrile;
In
1,2-dichloro-ethane;
at 15 ℃;
for 4h;
|
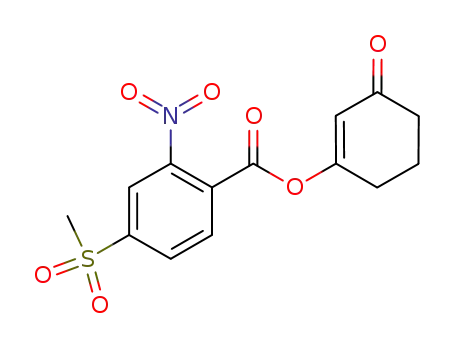
3-(4'-methylsulfonyl-2'-nitro-benzoyloxy)-2-cyclohexene-1-one


mesotrione
| Conditions | Yield |
|---|---|
|
With
caesium carbonate; 1,8-diazabicyclo[5.4.0]undec-7-ene;
at 20 - 30 ℃;
for 2h;
Reagent/catalyst;
Temperature;
|
95% |
|
With
6-chloro-7H-purine;
at 60 - 65 ℃;
for 4h;
|
92.3% |
|
With
triethylamine; 2-hydroxy-2-methylpropanenitrile;
In
acetonitrile;
at 20 ℃;
for 10h;
Inert atmosphere;
|
54% |
|
With
2-hydroxy-2-methylpropanenitrile;
|
|
|
With
2-hydroxy-2-methylpropanenitrile;
|
|
|
With
benzyl triethylammonium azide; triethylamine;
at 50 ℃;
Reagent/catalyst;
Temperature;
Solvent;
|
32 g |
|
With
triethylamine; 2-hydroxy-2-methylpropanenitrile;
at 20 - 25 ℃;
for 5h;
Reagent/catalyst;
|
|
|
3-(4'-methylsulfonyl-2'-nitro-benzoyloxy)-2-cyclohexene-1-one;
With
triethylamine;
In
dichloromethane;
at 30 ℃;
for 0.25h;
With
2-hydroxy-2-methylpropanenitrile;
In
dichloromethane;
at 30 ℃;
for 3h;
|
305 g |
|
With
3-cyano-3-hydroxy-1-propene;
In
1,2-dichloro-ethane;
at 55 - 60 ℃;
for 4h;
Temperature;
Reagent/catalyst;
Solvent;
|

3-(4'-methylsulfonyl-2'-nitro-benzoyloxy)-2-cyclohexene-1-one

1,3-cylohexanedione
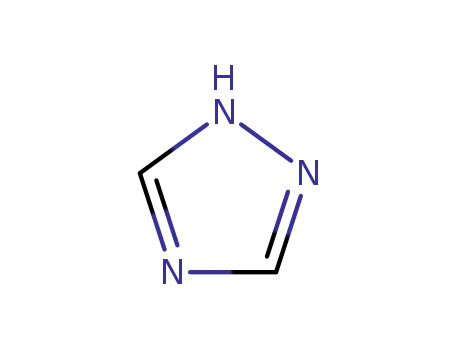
1,2,4-Triazole
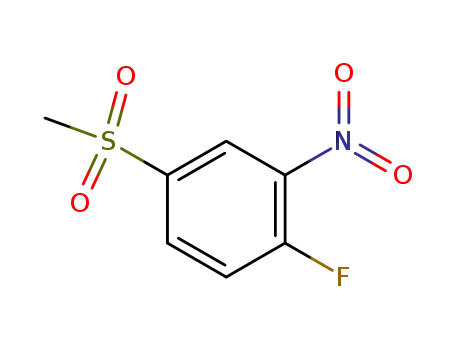
1-fluoro-4-methanesulfonyl-2-nitrobenzene
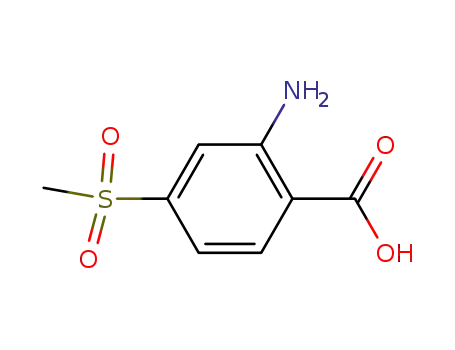
2-amino-4-(methylsulfonyl)benzoic acid
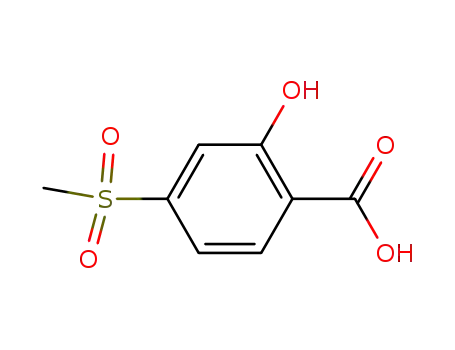
2-hydroxy-4-(methylsulfonyl)-2-nitrobenzoic acid
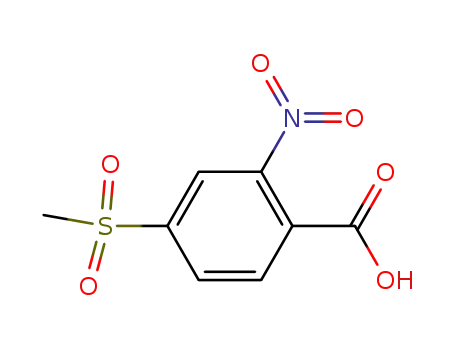
4-methanesulfonyl-2-nitro-benzoic acid
CAS:115473-15-9
CAS:1173-88-2
CAS:455943-61-0
CAS:75621-03-3