- +86-0533-2185556
- WhatsApp: +86 15965530500
- admin@hangyubiotech.com
Your Location:Home >Products >API >15690-57-0
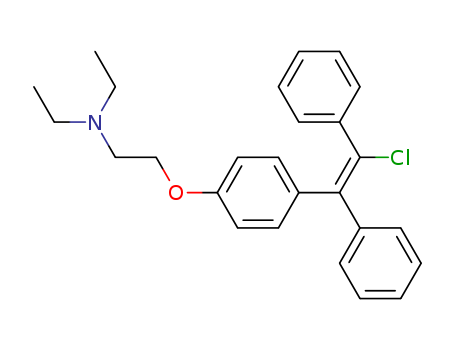

pd_meltingpoint:149.0-150.5°
Purity:99%
InChI:InChI=1/C26H28ClNO/c1-3-28(4-2)19-20-29-24-17-15-22(16-18-24)25(21-11-7-5-8-12-21)26(27)23-13-9-6-10-14-23/h5-18H,3-4,19-20H2,1-2H3/b26-25+
A TEMPO catalyzed cross-dihalogenation r...
Stereoselective synthesis of zuclomiphen...
The present invention provides salts of ...
The present invention provides processes...
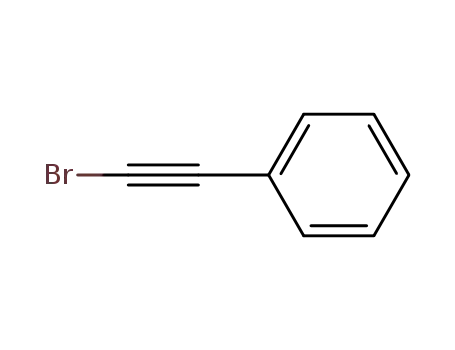
1-Bromo-2-phenylacetylene

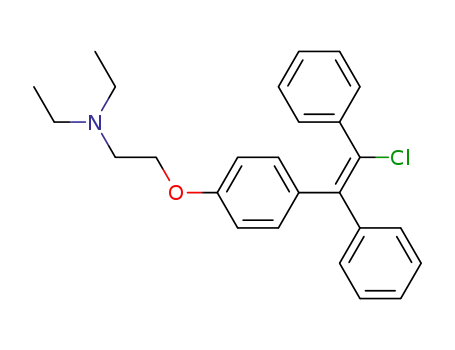
clomiphene

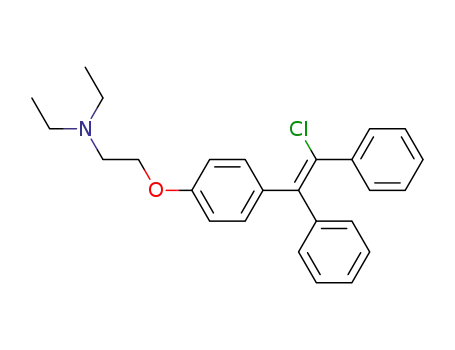
Zuclomiphene
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 5 steps
1: N-iodo-succinimide; N-chloro-succinimide / 1,2-dichloro-ethane / 24 h / 30 °C / Sealed tube
2: palladium diacetate; tricyclohexylphosphine; potassium hydroxide / tetrahydrofuran; water / 2 h / 80 °C / Inert atmosphere
3: potassium hydroxide; tetrakis(triphenylphosphine) palladium(0) / tetrahydrofuran; methanol / 12 h / 110 °C / Schlenk technique
4: boron tribromide / dichloromethane / 25 h / -78 - -20 °C
5: potassium carbonate / acetone / 1 h / 60 °C
With N-chloro-succinimide; N-iodo-succinimide; tetrakis(triphenylphosphine) palladium(0); palladium diacetate; boron tribromide; potassium carbonate; potassium hydroxide; tricyclohexylphosphine; In tetrahydrofuran; methanol; dichloromethane; water; 1,2-dichloro-ethane; acetone;
|
|
|
Multi-step reaction with 5 steps
1: 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical; N-iodo-succinimide; N-chloro-succinimide / 1,2-dichloro-ethane / 24 h / 30 °C / Sealed tube
2: palladium diacetate; tricyclohexylphosphine; potassium hydroxide / tetrahydrofuran; water / 2 h / 80 °C / Inert atmosphere
3: potassium hydroxide; tetrakis(triphenylphosphine) palladium(0) / tetrahydrofuran; methanol / 12 h / 110 °C / Schlenk technique
4: boron tribromide / dichloromethane / 25 h / -78 - -20 °C
5: potassium carbonate / acetone / 1 h / 60 °C
With N-chloro-succinimide; N-iodo-succinimide; tetrakis(triphenylphosphine) palladium(0); 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical; palladium diacetate; boron tribromide; potassium carbonate; potassium hydroxide; tricyclohexylphosphine; In tetrahydrofuran; methanol; dichloromethane; water; 1,2-dichloro-ethane; acetone;
|
|
|
Multi-step reaction with 5 steps
1: 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical; N-iodo-succinimide; N-chloro-succinimide / 1,2-dichloro-ethane / 24 h / 30 °C / Sealed tube
2: palladium diacetate; tricyclohexylphosphine; potassium hydroxide / tetrahydrofuran; water / 2 h / 80 °C / Inert atmosphere
3: potassium hydroxide; tetrakis(triphenylphosphine) palladium(0) / tetrahydrofuran; methanol / 12 h / 110 °C / Schlenk technique
4: boron tribromide / dichloromethane / 25 h / -78 - -20 °C
5: potassium carbonate / acetone / 1 h / 60 °C
With N-chloro-succinimide; N-iodo-succinimide; tetrakis(triphenylphosphine) palladium(0); 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical; palladium diacetate; boron tribromide; potassium carbonate; potassium hydroxide; tricyclohexylphosphine; In tetrahydrofuran; methanol; dichloromethane; water; 1,2-dichloro-ethane; acetone;
|
|
|
Multi-step reaction with 5 steps
1: 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical; N-iodo-succinimide; N-chloro-succinimide / toluene / 24 h / 30 °C / Sealed tube
2: palladium diacetate; tricyclohexylphosphine; potassium hydroxide / tetrahydrofuran; water / 2 h / 80 °C / Inert atmosphere
3: potassium hydroxide; tetrakis(triphenylphosphine) palladium(0) / tetrahydrofuran; methanol / 12 h / 110 °C / Schlenk technique
4: boron tribromide / dichloromethane / 25 h / -78 - -20 °C
5: potassium carbonate / acetone / 1 h / 60 °C
With N-chloro-succinimide; N-iodo-succinimide; tetrakis(triphenylphosphine) palladium(0); 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical; palladium diacetate; boron tribromide; potassium carbonate; potassium hydroxide; tricyclohexylphosphine; In tetrahydrofuran; methanol; dichloromethane; water; acetone; toluene;
|
|
|
Multi-step reaction with 5 steps
1: N-iodo-succinimide; N-chloro-succinimide; TEMPOL / 1,2-dichloro-ethane / 24 h / 30 °C / Sealed tube
2: palladium diacetate; tricyclohexylphosphine; potassium hydroxide / tetrahydrofuran; water / 2 h / 80 °C / Inert atmosphere
3: potassium hydroxide; tetrakis(triphenylphosphine) palladium(0) / tetrahydrofuran; methanol / 12 h / 110 °C / Schlenk technique
4: boron tribromide / dichloromethane / 25 h / -78 - -20 °C
5: potassium carbonate / acetone / 1 h / 60 °C
With N-chloro-succinimide; N-iodo-succinimide; tetrakis(triphenylphosphine) palladium(0); TEMPOL; palladium diacetate; boron tribromide; potassium carbonate; potassium hydroxide; tricyclohexylphosphine; In tetrahydrofuran; methanol; dichloromethane; water; 1,2-dichloro-ethane; acetone;
|
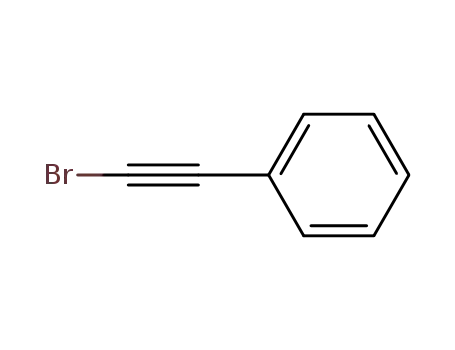
1-Bromo-2-phenylacetylene


clomiphene


Zuclomiphene
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 5 steps
1: N-iodo-succinimide; N-chloro-succinimide / 1,2-dichloro-ethane / 24 h / 30 °C / Sealed tube
2: palladium diacetate; tricyclohexylphosphine; potassium hydroxide / tetrahydrofuran; water / 2 h / 80 °C / Inert atmosphere
3: potassium hydroxide; tetrakis(triphenylphosphine) palladium(0) / tetrahydrofuran; methanol / 12 h / 110 °C / Schlenk technique
4: boron tribromide / dichloromethane / 25 h / -78 - -20 °C
5: potassium carbonate / acetone / 1 h / 60 °C
With N-chloro-succinimide; N-iodo-succinimide; tetrakis(triphenylphosphine) palladium(0); palladium diacetate; boron tribromide; potassium carbonate; potassium hydroxide; tricyclohexylphosphine; In tetrahydrofuran; methanol; dichloromethane; water; 1,2-dichloro-ethane; acetone;
|
|
|
Multi-step reaction with 5 steps
1: 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical; N-iodo-succinimide; N-chloro-succinimide / 1,2-dichloro-ethane / 24 h / 30 °C / Sealed tube
2: palladium diacetate; tricyclohexylphosphine; potassium hydroxide / tetrahydrofuran; water / 2 h / 80 °C / Inert atmosphere
3: potassium hydroxide; tetrakis(triphenylphosphine) palladium(0) / tetrahydrofuran; methanol / 12 h / 110 °C / Schlenk technique
4: boron tribromide / dichloromethane / 25 h / -78 - -20 °C
5: potassium carbonate / acetone / 1 h / 60 °C
With N-chloro-succinimide; N-iodo-succinimide; tetrakis(triphenylphosphine) palladium(0); 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical; palladium diacetate; boron tribromide; potassium carbonate; potassium hydroxide; tricyclohexylphosphine; In tetrahydrofuran; methanol; dichloromethane; water; 1,2-dichloro-ethane; acetone;
|
|
|
Multi-step reaction with 5 steps
1: 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical; N-iodo-succinimide; N-chloro-succinimide / 1,2-dichloro-ethane / 24 h / 30 °C / Sealed tube
2: palladium diacetate; tricyclohexylphosphine; potassium hydroxide / tetrahydrofuran; water / 2 h / 80 °C / Inert atmosphere
3: potassium hydroxide; tetrakis(triphenylphosphine) palladium(0) / tetrahydrofuran; methanol / 12 h / 110 °C / Schlenk technique
4: boron tribromide / dichloromethane / 25 h / -78 - -20 °C
5: potassium carbonate / acetone / 1 h / 60 °C
With N-chloro-succinimide; N-iodo-succinimide; tetrakis(triphenylphosphine) palladium(0); 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical; palladium diacetate; boron tribromide; potassium carbonate; potassium hydroxide; tricyclohexylphosphine; In tetrahydrofuran; methanol; dichloromethane; water; 1,2-dichloro-ethane; acetone;
|
|
|
Multi-step reaction with 5 steps
1: 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical; N-iodo-succinimide; N-chloro-succinimide / toluene / 24 h / 30 °C / Sealed tube
2: palladium diacetate; tricyclohexylphosphine; potassium hydroxide / tetrahydrofuran; water / 2 h / 80 °C / Inert atmosphere
3: potassium hydroxide; tetrakis(triphenylphosphine) palladium(0) / tetrahydrofuran; methanol / 12 h / 110 °C / Schlenk technique
4: boron tribromide / dichloromethane / 25 h / -78 - -20 °C
5: potassium carbonate / acetone / 1 h / 60 °C
With N-chloro-succinimide; N-iodo-succinimide; tetrakis(triphenylphosphine) palladium(0); 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical; palladium diacetate; boron tribromide; potassium carbonate; potassium hydroxide; tricyclohexylphosphine; In tetrahydrofuran; methanol; dichloromethane; water; acetone; toluene;
|
|
|
Multi-step reaction with 5 steps
1: N-iodo-succinimide; N-chloro-succinimide; TEMPOL / 1,2-dichloro-ethane / 24 h / 30 °C / Sealed tube
2: palladium diacetate; tricyclohexylphosphine; potassium hydroxide / tetrahydrofuran; water / 2 h / 80 °C / Inert atmosphere
3: potassium hydroxide; tetrakis(triphenylphosphine) palladium(0) / tetrahydrofuran; methanol / 12 h / 110 °C / Schlenk technique
4: boron tribromide / dichloromethane / 25 h / -78 - -20 °C
5: potassium carbonate / acetone / 1 h / 60 °C
With N-chloro-succinimide; N-iodo-succinimide; tetrakis(triphenylphosphine) palladium(0); TEMPOL; palladium diacetate; boron tribromide; potassium carbonate; potassium hydroxide; tricyclohexylphosphine; In tetrahydrofuran; methanol; dichloromethane; water; 1,2-dichloro-ethane; acetone;
|
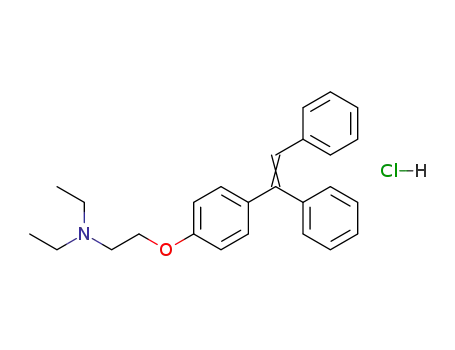
N,N-diethyl-2-[4-(1,2-diphenylvinyl)phenoxy]ethylamine hydrochloride
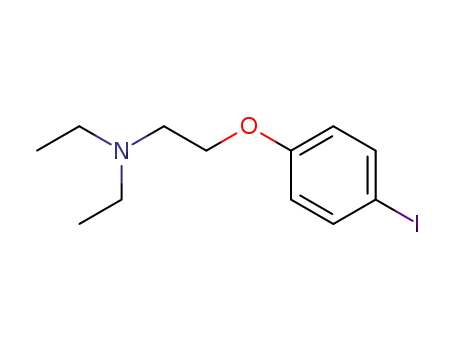
4-<2-(diethylamino)ethoxy>iodobenzene
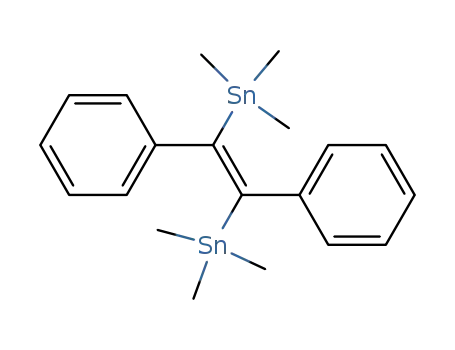
(E)-bis(trimethylstannyl)stilbene
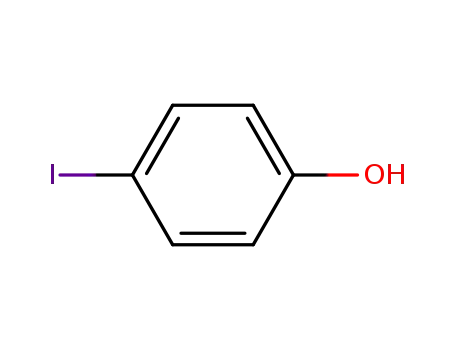
p-Iodophenol
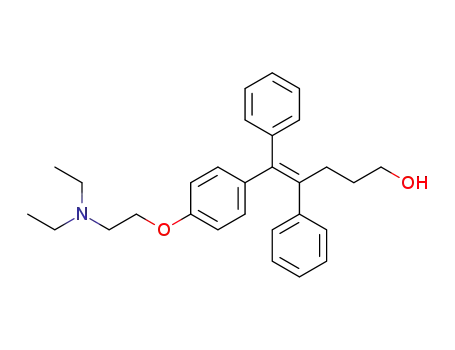
(Z)-1-<4-(2-diethylaminoethoxy)phenyl>-1,2-diphenyl-1-penten-5-ol
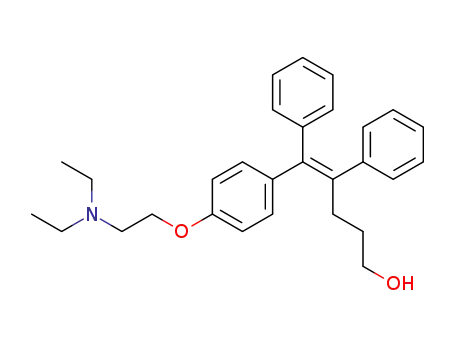
(E)-1-<4-(2-diethylaminoethoxy)phenyl>-1,2-diphenyl-1-penten-5-ol
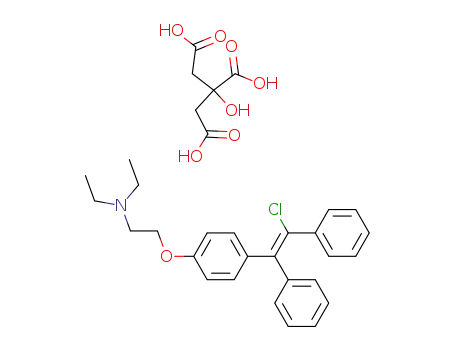
cis-clomiphene citrate
clomiphene citrate
CAS:115473-15-9
CAS:118685-33-9