- +86-0533-2185556
- WhatsApp: +86 15965530500
- admin@hangyubiotech.com
Your Location:Home >Products >API >709031-45-8

Purity:99%
Described is an improved and industriall...
All eight stereoisomers of saxagliptin h...
The commercial-scale synthesis of the DP...
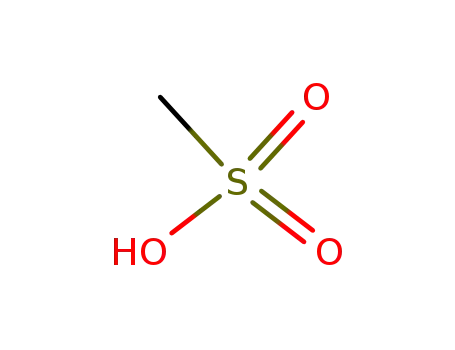
methanesulfonic acid

![[1S-(1α,3β,5α)]-3-(aminocarbonyl)-2-azabicyclo[3.1.0]hexane-2-carboxylic acid 1,1-dimethylethyl ester](/upload/2025/4/9b0c03a8-29b0-4bda-953a-d3238a4d25d7.png)
[1S-(1α,3β,5α)]-3-(aminocarbonyl)-2-azabicyclo[3.1.0]hexane-2-carboxylic acid 1,1-dimethylethyl ester

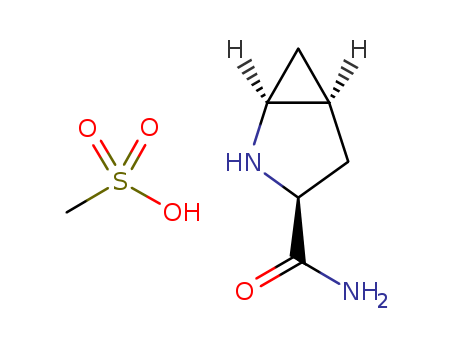
![(1S,3S,5S)-2-azabicyclo[3.1.0]hexane-3-carboxamide methane sulphonic acid](/upload/2025/4/395a1a05-e99a-453e-a79e-0a6798c177f7.png)
(1S,3S,5S)-2-azabicyclo[3.1.0]hexane-3-carboxamide methane sulphonic acid
| Conditions | Yield |
|---|---|
|
In isopropyl alcohol; at 60 - 70 ℃; for 5h;
|
96.7% |
|
In isopropyl alcohol; at 55 - 65 ℃; for 3.5h; Large scale reaction;
|
94% |
|
In isopropyl alcohol; at 60 ℃;
|
80% |
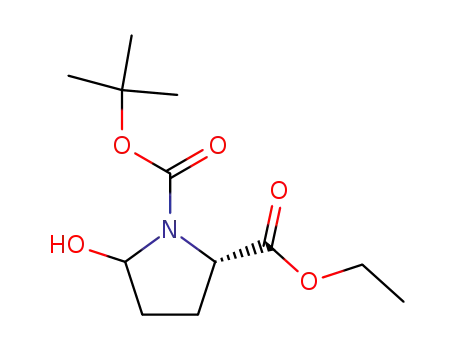
5-hydroxy-pyrrolidine-1,2-dicarboxylic acid 1-tert-butyl ester 2-ethyl ester

![(1S,3S,5S)-2-azabicyclo[3.1.0]hexane-3-carboxamide methane sulphonic acid](/upload/2025/4/395a1a05-e99a-453e-a79e-0a6798c177f7.png)
(1S,3S,5S)-2-azabicyclo[3.1.0]hexane-3-carboxamide methane sulphonic acid
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 5 steps
1: dmap; trifluoroacetic anhydride; N-ethyl-N,N-diisopropylamine
2: water; lithium hydroxide / ethanol
3: ammonia; N-ethyl-N,N-diisopropylamine; methanesulfonyl chloride / tetrahydrofuran
4: diethylzinc / 1,2-dimethoxyethane; dichloromethane / -30 - 20 °C
5: isopropyl alcohol / 60 °C
With dmap; ammonia; water; diethylzinc; methanesulfonyl chloride; N-ethyl-N,N-diisopropylamine; trifluoroacetic anhydride; lithium hydroxide; In tetrahydrofuran; 1,2-dimethoxyethane; ethanol; dichloromethane; isopropyl alcohol; 4: |Simmons-Smith Cyclopropanation;
|

methanesulfonic acid
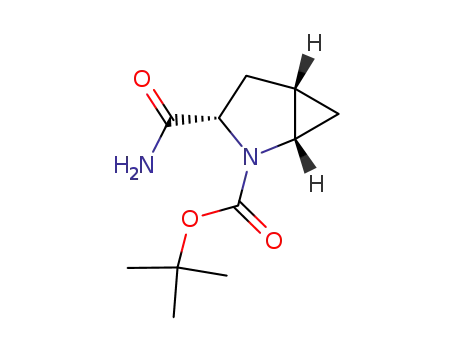
[1S-(1α,3β,5α)]-3-(aminocarbonyl)-2-azabicyclo[3.1.0]hexane-2-carboxylic acid 1,1-dimethylethyl ester

5-hydroxy-pyrrolidine-1,2-dicarboxylic acid 1-tert-butyl ester 2-ethyl ester
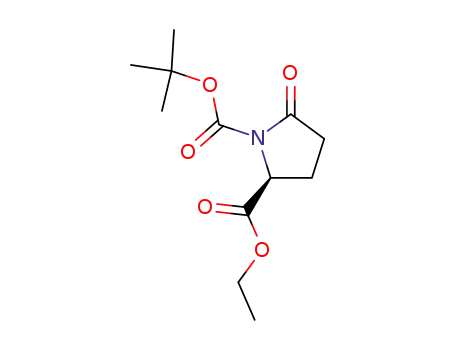
(S)-ethyl N-tert-butoxycarbonylpyroglutamate
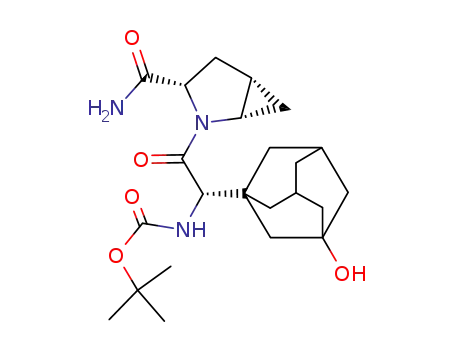
tert-butyl [(1S)-2-[(1S,3S,5S)-3-carbamoyl-2-azabicyclo[3.1.0]hex-2-yl]-1-(3-hydroxytricyclo[3.3.1.13'7]dec-1-yl)-2-oxoethyl]carbamate
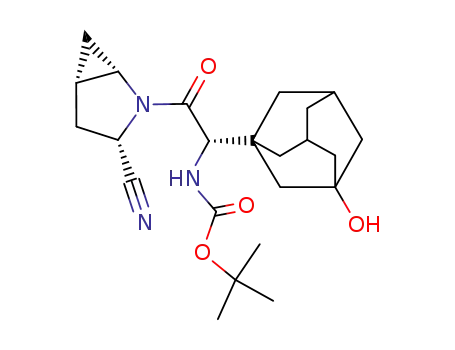
3-cyano-(αS)-α-(3-hydroxytricyclo[3.3.1.13,7]dec-1-yl)-β-oxo-(1S,3S,5S)-2-azabicyclo[3.1.0]hexane-2-ethanecarbamic acid 1,1-dimethylethyl ester
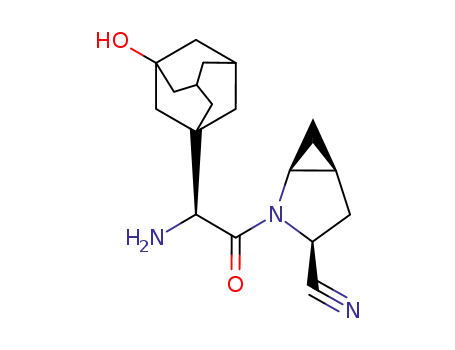
saxagliptin
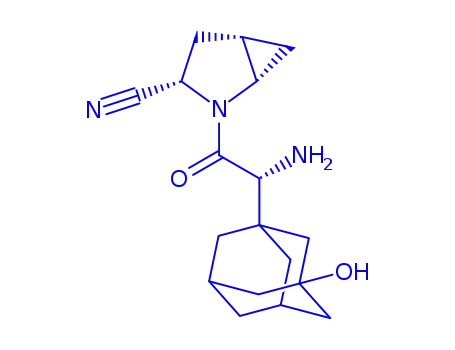
(R)-3-hydroxyadamantylglycine-L-cis-4,5-methanoprolinenitrile
CAS:118685-33-9
CAS:66104-23-2
CAS:221615-75-4