- +86-0533-2185556
- WhatsApp: +86 15965530500
- admin@hangyubiotech.com
Your Location:Home >Products >small-molecule inhibitor >1923833-60-6
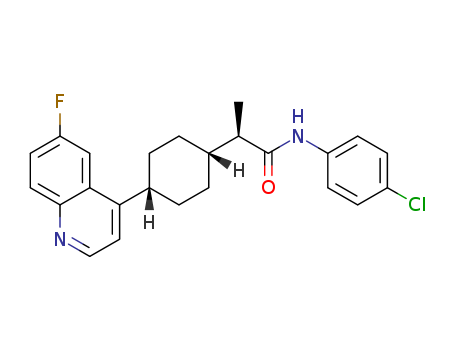

Purity:99%
The process development and the kilogram...
Formulated and/or co-formulated liposome...
Compounds that modulate the oxidoreducta...
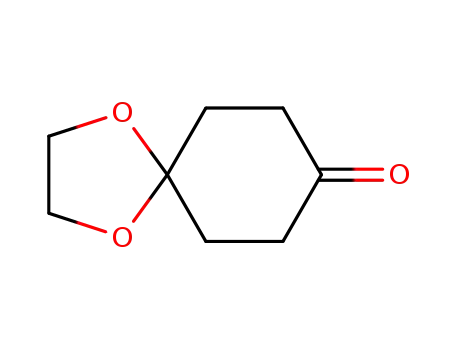
cyclohexanedione monoethylene ketal

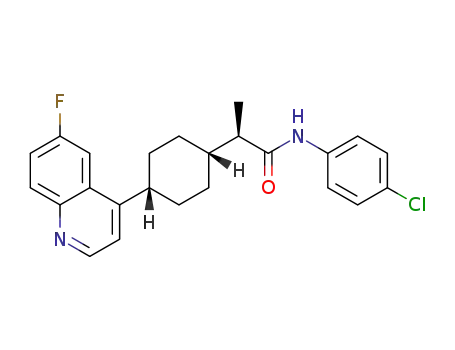
(R)-N-(4-chlorophenyl)-2-((1S,4S)-4-(6-fluoroquinolin-4-yl)cyclohexyl)propanamide
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 12 steps
1.1: sodium hydride / tetrahydrofuran; mineral oil / 0.5 h / 0 - 20 °C
1.2: 0.5 h / 0 - 20 °C
2.1: palladium 10% on activated carbon; hydrogen / ethyl acetate / 4 h / 2585.81 Torr / Inert atmosphere
3.1: hydrogenchloride / water; acetone / 2 h / Reflux
4.1: 2,6-di-tert-butyl-4-methylpyridine / dichloromethane / 20 °C / Inert atmosphere
5.1: potassium acetate; dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2 / 1,4-dioxane / 16 h / 80 °C / Inert atmosphere
6.1: tetrakis(triphenylphosphine) palladium(0); potassium carbonate / water; 1,4-dioxane / 16 h / 100 °C / Sealed tube; Inert atmosphere
7.1: palladium on activated charcoal; ammonium formate / methanol / 1 h / Reflux
8.1: lithium hydroxide; water / ethanol / 6 h / 50 °C
9.1: triethylamine; pivaloyl chloride / tetrahydrofuran / 1.25 h / -78 - 0 °C / Inert atmosphere
9.2: 3.25 h / -78 - 20 °C
10.1: sodium hexamethyldisilazane / tetrahydrofuran / 0.17 h / -50 °C
10.2: -50 - -20 °C
11.1: lithium hydroxide; dihydrogen peroxide / water; tetrahydrofuran / 0 - 20 °C
12.1: propylphosphonic anhydride; pyridine / ethyl acetate / 0.08 h / 20 °C
12.2: 20 °C
With
pyridine; hydrogenchloride; tetrakis(triphenylphosphine) palladium(0); 2,6-di-tert-butyl-4-methylpyridine; dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2; palladium 10% on activated carbon; palladium on activated charcoal; propylphosphonic anhydride; water; hydrogen; dihydrogen peroxide; ammonium formate; potassium acetate; sodium hexamethyldisilazane; pivaloyl chloride; sodium hydride; potassium carbonate; triethylamine; lithium hydroxide;
In
tetrahydrofuran; 1,4-dioxane; methanol; ethanol; dichloromethane; water; ethyl acetate; acetone; mineral oil;
|
|
|
Multi-step reaction with 12 steps
1.1: n-butyllithium / tert-butyl methyl ether / 4 h / -75 - -10 °C / Industrial scale
2.1: thionyl chloride; pyridine / 0.5 h / 5 - 15 °C / Industrial scale
3.1: hydrogen; palladium 10% on activated carbon / tetrahydrofuran / 80 h / 30 - 35 °C / 1125.11 - 1500.15 Torr / Industrial scale
4.1: hydrogenchloride / ethanol; water / 8 h / 50 - 55 °C / Industrial scale
5.1: cerium(III) chloride heptahydrate; sodium tetrahydroborate / ethanol / 17 h / 10 °C / Industrial scale
6.1: pyridine / acetonitrile / 23 h / 10 - 20 °C / Industrial scale
7.1: sodium t-butanolate / 1-methyl-pyrrolidin-2-one / 16 h / 85 °C
8.1: methanesulfonic acid / water; sulfolane / 14 h / 55 - 105 °C / Industrial scale
8.2: 25 °C / Industrial scale
9.1: triethylamine; lithium chloride; pivaloyl chloride / tetrahydrofuran / 8 h / 25 °C / Industrial scale
10.1: tetrahydrofuran / -20 °C / Industrial scale
11.1: dihydrogen peroxide; lithium hydroxide / tetrahydrofuran; water / 25 °C / Industrial scale
12.1: N,N,N',N'-tetramethylchloroformamidinium hexafluorophosphate; 1-methyl-1H-imidazole / acetonitrile / 3 h / 20 °C / Industrial scale
With
pyridine; 1-methyl-1H-imidazole; hydrogenchloride; sodium tetrahydroborate; n-butyllithium; thionyl chloride; methanesulfonic acid; cerium(III) chloride heptahydrate; palladium 10% on activated carbon; hydrogen; dihydrogen peroxide; pivaloyl chloride; N,N,N',N'-tetramethylchloroformamidinium hexafluorophosphate; triethylamine; lithium chloride; lithium hydroxide; sodium t-butanolate;
In
tetrahydrofuran; 1-methyl-pyrrolidin-2-one; sulfolane; ethanol; tert-butyl methyl ether; water; acetonitrile;
|
|
|
Multi-step reaction with 12 steps
1.1: n-butyllithium / tert-butyl methyl ether / 4 h / -75 - -10 °C / Industrial scale
2.1: thionyl chloride; pyridine / 0.5 h / 5 - 15 °C / Industrial scale
3.1: hydrogen; palladium 10% on activated carbon / tetrahydrofuran / 80 h / 30 - 35 °C / 1125.11 - 1500.15 Torr / Industrial scale
4.1: hydrogenchloride / ethanol; water / 8 h / 50 - 55 °C / Industrial scale
5.1: cerium(III) chloride heptahydrate; sodium tetrahydroborate / ethanol / 17 h / 10 °C / Industrial scale
6.1: pyridine / acetonitrile / 23 h / 10 - 20 °C / Industrial scale
7.1: sodium tert-pentoxide / toluene / 12 h / 35 - 85 °C / Industrial scale
8.1: methanesulfonic acid / water; sulfolane / 14 h / 55 - 105 °C / Industrial scale
8.2: 25 °C / Industrial scale
9.1: triethylamine; lithium chloride; pivaloyl chloride / tetrahydrofuran / 8 h / 25 °C / Industrial scale
10.1: tetrahydrofuran / -20 °C / Industrial scale
11.1: dihydrogen peroxide; lithium hydroxide / tetrahydrofuran; water / 25 °C / Industrial scale
12.1: N,N,N',N'-tetramethylchloroformamidinium hexafluorophosphate; 1-methyl-1H-imidazole / acetonitrile / 3 h / 20 °C / Industrial scale
With
pyridine; 1-methyl-1H-imidazole; hydrogenchloride; sodium tetrahydroborate; n-butyllithium; thionyl chloride; methanesulfonic acid; cerium(III) chloride heptahydrate; palladium 10% on activated carbon; hydrogen; dihydrogen peroxide; pivaloyl chloride; sodium tert-pentoxide; N,N,N',N'-tetramethylchloroformamidinium hexafluorophosphate; triethylamine; lithium chloride; lithium hydroxide;
In
tetrahydrofuran; sulfolane; ethanol; tert-butyl methyl ether; water; toluene; acetonitrile;
|
|
|
Multi-step reaction with 12 steps
1: n-butyllithium / tert-butyl methyl ether / 4 h / -75 - -10 °C / Industrial scale
2: thionyl chloride; pyridine / 0.5 h / 5 - 15 °C / Industrial scale
3: hydrogen; palladium 10% on activated carbon / tetrahydrofuran / 80 h / 30 - 35 °C / 1125.11 - 1500.15 Torr / Industrial scale
4: hydrogenchloride / ethanol; water / 8 h / 50 - 55 °C / Industrial scale
5: cerium(III) chloride heptahydrate; sodium tetrahydroborate / ethanol / 17 h / 10 °C / Industrial scale
6: pyridine / acetonitrile / 23 h / 10 - 20 °C / Industrial scale
7: sodium t-butanolate / 1-methyl-pyrrolidin-2-one / 16 h / 85 °C
8: acetic acid / water / 130 °C
9: triethylamine; lithium chloride; pivaloyl chloride / tetrahydrofuran / 8 h / 25 °C / Industrial scale
10: tetrahydrofuran / -20 °C / Industrial scale
11: dihydrogen peroxide; lithium hydroxide / tetrahydrofuran; water / 25 °C / Industrial scale
12: N,N,N',N'-tetramethylchloroformamidinium hexafluorophosphate; 1-methyl-1H-imidazole / acetonitrile / 3 h / 20 °C / Industrial scale
With
pyridine; 1-methyl-1H-imidazole; hydrogenchloride; sodium tetrahydroborate; n-butyllithium; thionyl chloride; cerium(III) chloride heptahydrate; palladium 10% on activated carbon; hydrogen; dihydrogen peroxide; pivaloyl chloride; N,N,N',N'-tetramethylchloroformamidinium hexafluorophosphate; acetic acid; triethylamine; lithium chloride; lithium hydroxide; sodium t-butanolate;
In
tetrahydrofuran; 1-methyl-pyrrolidin-2-one; ethanol; tert-butyl methyl ether; water; acetonitrile;
|
|
|
Multi-step reaction with 12 steps
1: n-butyllithium / tert-butyl methyl ether / 4 h / -75 - -10 °C / Industrial scale
2: thionyl chloride; pyridine / 0.5 h / 5 - 15 °C / Industrial scale
3: hydrogen; palladium 10% on activated carbon / tetrahydrofuran / 80 h / 30 - 35 °C / 1125.11 - 1500.15 Torr / Industrial scale
4: hydrogenchloride / ethanol; water / 8 h / 50 - 55 °C / Industrial scale
5: cerium(III) chloride heptahydrate; sodium tetrahydroborate / ethanol / 17 h / 10 °C / Industrial scale
6: pyridine / acetonitrile / 23 h / 10 - 20 °C / Industrial scale
7: sodium tert-pentoxide / toluene / 12 h / 35 - 85 °C / Industrial scale
8: acetic acid / water / 130 °C
9: triethylamine; lithium chloride; pivaloyl chloride / tetrahydrofuran / 8 h / 25 °C / Industrial scale
10: tetrahydrofuran / -20 °C / Industrial scale
11: dihydrogen peroxide; lithium hydroxide / tetrahydrofuran; water / 25 °C / Industrial scale
12: N,N,N',N'-tetramethylchloroformamidinium hexafluorophosphate; 1-methyl-1H-imidazole / acetonitrile / 3 h / 20 °C / Industrial scale
With
pyridine; 1-methyl-1H-imidazole; hydrogenchloride; sodium tetrahydroborate; n-butyllithium; thionyl chloride; cerium(III) chloride heptahydrate; palladium 10% on activated carbon; hydrogen; dihydrogen peroxide; pivaloyl chloride; sodium tert-pentoxide; N,N,N',N'-tetramethylchloroformamidinium hexafluorophosphate; acetic acid; triethylamine; lithium chloride; lithium hydroxide;
In
tetrahydrofuran; ethanol; tert-butyl methyl ether; water; toluene; acetonitrile;
|
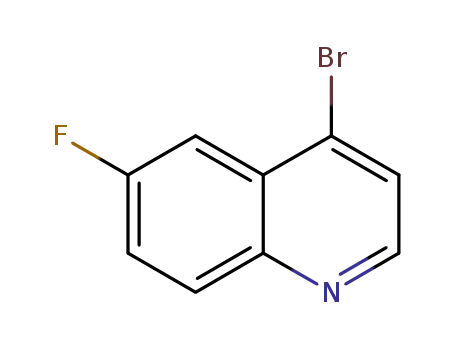
4-bromine-6-fluoroquinoline


(R)-N-(4-chlorophenyl)-2-((1S,4S)-4-(6-fluoroquinolin-4-yl)cyclohexyl)propanamide
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 12 steps
1.1: n-butyllithium / tert-butyl methyl ether / 4 h / -75 - -10 °C / Industrial scale
2.1: thionyl chloride; pyridine / 0.5 h / 5 - 15 °C / Industrial scale
3.1: hydrogen; palladium 10% on activated carbon / tetrahydrofuran / 80 h / 30 - 35 °C / 1125.11 - 1500.15 Torr / Industrial scale
4.1: hydrogenchloride / ethanol; water / 8 h / 50 - 55 °C / Industrial scale
5.1: cerium(III) chloride heptahydrate; sodium tetrahydroborate / ethanol / 17 h / 10 °C / Industrial scale
6.1: pyridine / acetonitrile / 23 h / 10 - 20 °C / Industrial scale
7.1: sodium t-butanolate / 1-methyl-pyrrolidin-2-one / 16 h / 85 °C
8.1: methanesulfonic acid / water; sulfolane / 14 h / 55 - 105 °C / Industrial scale
8.2: 25 °C / Industrial scale
9.1: triethylamine; lithium chloride; pivaloyl chloride / tetrahydrofuran / 8 h / 25 °C / Industrial scale
10.1: tetrahydrofuran / -20 °C / Industrial scale
11.1: dihydrogen peroxide; lithium hydroxide / tetrahydrofuran; water / 25 °C / Industrial scale
12.1: N,N,N',N'-tetramethylchloroformamidinium hexafluorophosphate; 1-methyl-1H-imidazole / acetonitrile / 3 h / 20 °C / Industrial scale
With
pyridine; 1-methyl-1H-imidazole; hydrogenchloride; sodium tetrahydroborate; n-butyllithium; thionyl chloride; methanesulfonic acid; cerium(III) chloride heptahydrate; palladium 10% on activated carbon; hydrogen; dihydrogen peroxide; pivaloyl chloride; N,N,N',N'-tetramethylchloroformamidinium hexafluorophosphate; triethylamine; lithium chloride; lithium hydroxide; sodium t-butanolate;
In
tetrahydrofuran; 1-methyl-pyrrolidin-2-one; sulfolane; ethanol; tert-butyl methyl ether; water; acetonitrile;
|
|
|
Multi-step reaction with 12 steps
1.1: n-butyllithium / tert-butyl methyl ether / 4 h / -75 - -10 °C / Industrial scale
2.1: thionyl chloride; pyridine / 0.5 h / 5 - 15 °C / Industrial scale
3.1: hydrogen; palladium 10% on activated carbon / tetrahydrofuran / 80 h / 30 - 35 °C / 1125.11 - 1500.15 Torr / Industrial scale
4.1: hydrogenchloride / ethanol; water / 8 h / 50 - 55 °C / Industrial scale
5.1: cerium(III) chloride heptahydrate; sodium tetrahydroborate / ethanol / 17 h / 10 °C / Industrial scale
6.1: pyridine / acetonitrile / 23 h / 10 - 20 °C / Industrial scale
7.1: sodium tert-pentoxide / toluene / 12 h / 35 - 85 °C / Industrial scale
8.1: methanesulfonic acid / water; sulfolane / 14 h / 55 - 105 °C / Industrial scale
8.2: 25 °C / Industrial scale
9.1: triethylamine; lithium chloride; pivaloyl chloride / tetrahydrofuran / 8 h / 25 °C / Industrial scale
10.1: tetrahydrofuran / -20 °C / Industrial scale
11.1: dihydrogen peroxide; lithium hydroxide / tetrahydrofuran; water / 25 °C / Industrial scale
12.1: N,N,N',N'-tetramethylchloroformamidinium hexafluorophosphate; 1-methyl-1H-imidazole / acetonitrile / 3 h / 20 °C / Industrial scale
With
pyridine; 1-methyl-1H-imidazole; hydrogenchloride; sodium tetrahydroborate; n-butyllithium; thionyl chloride; methanesulfonic acid; cerium(III) chloride heptahydrate; palladium 10% on activated carbon; hydrogen; dihydrogen peroxide; pivaloyl chloride; sodium tert-pentoxide; N,N,N',N'-tetramethylchloroformamidinium hexafluorophosphate; triethylamine; lithium chloride; lithium hydroxide;
In
tetrahydrofuran; sulfolane; ethanol; tert-butyl methyl ether; water; toluene; acetonitrile;
|
|
|
Multi-step reaction with 12 steps
1: n-butyllithium / tert-butyl methyl ether / 4 h / -75 - -10 °C / Industrial scale
2: thionyl chloride; pyridine / 0.5 h / 5 - 15 °C / Industrial scale
3: hydrogen; palladium 10% on activated carbon / tetrahydrofuran / 80 h / 30 - 35 °C / 1125.11 - 1500.15 Torr / Industrial scale
4: hydrogenchloride / ethanol; water / 8 h / 50 - 55 °C / Industrial scale
5: cerium(III) chloride heptahydrate; sodium tetrahydroborate / ethanol / 17 h / 10 °C / Industrial scale
6: pyridine / acetonitrile / 23 h / 10 - 20 °C / Industrial scale
7: sodium t-butanolate / 1-methyl-pyrrolidin-2-one / 16 h / 85 °C
8: acetic acid / water / 130 °C
9: triethylamine; lithium chloride; pivaloyl chloride / tetrahydrofuran / 8 h / 25 °C / Industrial scale
10: tetrahydrofuran / -20 °C / Industrial scale
11: dihydrogen peroxide; lithium hydroxide / tetrahydrofuran; water / 25 °C / Industrial scale
12: N,N,N',N'-tetramethylchloroformamidinium hexafluorophosphate; 1-methyl-1H-imidazole / acetonitrile / 3 h / 20 °C / Industrial scale
With
pyridine; 1-methyl-1H-imidazole; hydrogenchloride; sodium tetrahydroborate; n-butyllithium; thionyl chloride; cerium(III) chloride heptahydrate; palladium 10% on activated carbon; hydrogen; dihydrogen peroxide; pivaloyl chloride; N,N,N',N'-tetramethylchloroformamidinium hexafluorophosphate; acetic acid; triethylamine; lithium chloride; lithium hydroxide; sodium t-butanolate;
In
tetrahydrofuran; 1-methyl-pyrrolidin-2-one; ethanol; tert-butyl methyl ether; water; acetonitrile;
|
|
|
Multi-step reaction with 12 steps
1: n-butyllithium / tert-butyl methyl ether / 4 h / -75 - -10 °C / Industrial scale
2: thionyl chloride; pyridine / 0.5 h / 5 - 15 °C / Industrial scale
3: hydrogen; palladium 10% on activated carbon / tetrahydrofuran / 80 h / 30 - 35 °C / 1125.11 - 1500.15 Torr / Industrial scale
4: hydrogenchloride / ethanol; water / 8 h / 50 - 55 °C / Industrial scale
5: cerium(III) chloride heptahydrate; sodium tetrahydroborate / ethanol / 17 h / 10 °C / Industrial scale
6: pyridine / acetonitrile / 23 h / 10 - 20 °C / Industrial scale
7: sodium tert-pentoxide / toluene / 12 h / 35 - 85 °C / Industrial scale
8: acetic acid / water / 130 °C
9: triethylamine; lithium chloride; pivaloyl chloride / tetrahydrofuran / 8 h / 25 °C / Industrial scale
10: tetrahydrofuran / -20 °C / Industrial scale
11: dihydrogen peroxide; lithium hydroxide / tetrahydrofuran; water / 25 °C / Industrial scale
12: N,N,N',N'-tetramethylchloroformamidinium hexafluorophosphate; 1-methyl-1H-imidazole / acetonitrile / 3 h / 20 °C / Industrial scale
With
pyridine; 1-methyl-1H-imidazole; hydrogenchloride; sodium tetrahydroborate; n-butyllithium; thionyl chloride; cerium(III) chloride heptahydrate; palladium 10% on activated carbon; hydrogen; dihydrogen peroxide; pivaloyl chloride; sodium tert-pentoxide; N,N,N',N'-tetramethylchloroformamidinium hexafluorophosphate; acetic acid; triethylamine; lithium chloride; lithium hydroxide;
In
tetrahydrofuran; ethanol; tert-butyl methyl ether; water; toluene; acetonitrile;
|
|
|
Multi-step reaction with 7 steps
1: caesium carbonate; [1,3-bis(2,6-diisopropylphenyl)imidazol-2-ylidene](3-chloropyridyl)palladium(ll) dichloride / 1,4-dioxane; water / 100 °C
2: palladium on activated charcoal; hydrogen
3: lithium hydroxide / water; ethanol / 50 °C
4: triethylamine; pivaloyl chloride / tetrahydrofuran / 50 °C
5: sodium hexamethyldisilazane / tetrahydrofuran / -50 °C
6: lithium hydroxide; dihydrogen peroxide / water; tetrahydrofuran / 0 °C
7: pyridine / ethyl acetate
With
pyridine; [1,3-bis(2,6-diisopropylphenyl)imidazol-2-ylidene](3-chloropyridyl)palladium(ll) dichloride; palladium on activated charcoal; hydrogen; dihydrogen peroxide; sodium hexamethyldisilazane; pivaloyl chloride; caesium carbonate; triethylamine; lithium hydroxide;
In
tetrahydrofuran; 1,4-dioxane; ethanol; water; ethyl acetate;
|
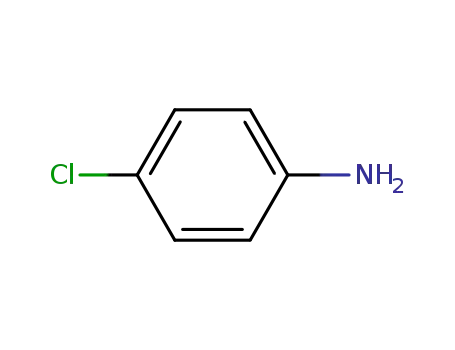
4-chloro-aniline
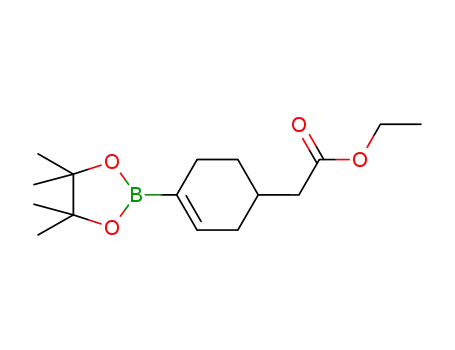
ethyl 2-(4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)cyclohex-3-en-1-yl)acetate
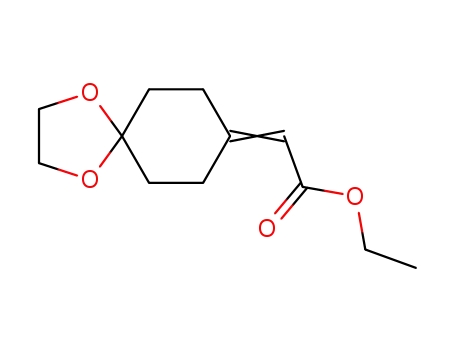
ethyl 1,4-dioxaspiro[4,5]dec-8-ylideneacetate
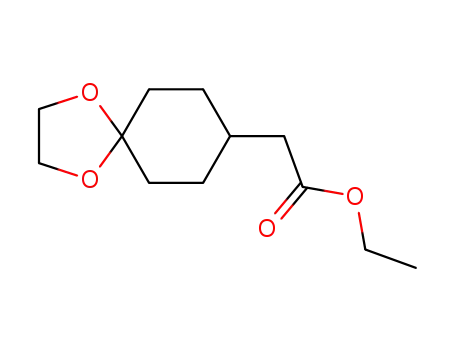
ethyl 1,4-dioxaspiro[4.5]dec-8-ylacetate
CAS:1642288-47-8
CAS:9002-01-1
CAS:2381-87-5
CAS:146062-44-4