- +86-0533-2185556
- WhatsApp: +86 15965530500
- admin@hangyubiotech.com
Your Location:Home >Products >small-molecule inhibitor >25554-84-1
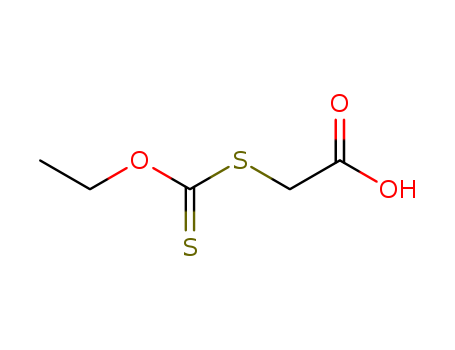

Purity:99%
|
Physical state |
Clear, colorless liquid |
|
Odor |
Strong sulfur-like |
|
Health hazards |
Corrosive to skin and eyes, respiratory irritation, harmful if ingested or inhaled |
|
Environmental impact |
Can cause damage to water sources if released |
|
General Description |
2-(Ethoxythioxomethylthio)acetic acid is a chemical compound with the molecular formula C6H10O4S3. It is a clear, colorless liquid with a strong sulfur-like odor and is commonly used as a pesticide intermediate and in the production of pharmaceuticals. It is also used as a reagent in organic synthesis and in the manufacturing of organic chemicals. This chemical compound has potential health hazards and should be handled with care, as it is corrosive to the skin and eyes and can cause respiratory irritation. Additionally, it may be harmful if ingested or inhaled and can cause environmental damage if released into water sources. |
InChI:InChI=1/C5H8O3S2/c1-2-8-5(9)10-3-4(6)7/h2-3H2,1H3,(H,6,7)
Radical functionalization of reduced gra...
Carbonyl sulfide (COS) is a gas that may...
-
N-substituted glycine N-thiocarboxyanhyd...
Biocompatible amphiphilic block copolyme...
Polymers with regulated alternating stru...
A library ofN-thiocarboxyanhydrides (NTA...
Mucus lines the moist cavities throughou...
A simple method for the introduction of ...
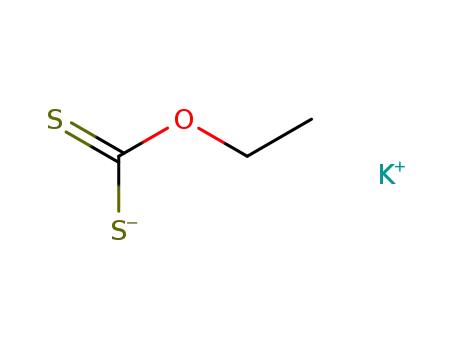
potassium ethyl xanthogenate

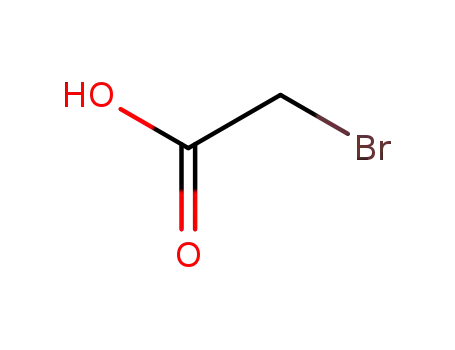
bromoacetic acid

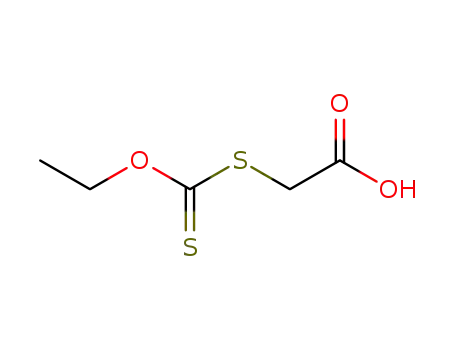
2-(ethoxycarbonothioylthio)acetic acid
| Conditions | Yield |
|---|---|
|
With
sodium hydroxide;
In
water monomer;
at 20 ℃;
for 24.0833h;
Cooling with ice;
|
81% |
|
In
acetone;
at 20 ℃;
|
75% |
|
In
water monomer;
at 0 - 20 ℃;
for 1h;
|
74% |
|
In
acetone;
at 20 ℃;
|
50% |
|
In
acetone;
at 20 ℃;
for 16.3333h;
|
33% |
|
In
acetone;
at 20 ℃;
for 16h;
|
33% |
|
In
tetrahydrofuran;
at 20 ℃;
for 48h;
|
30.4% |
|
In
tetrahydrofuran;
at 20 ℃;
|
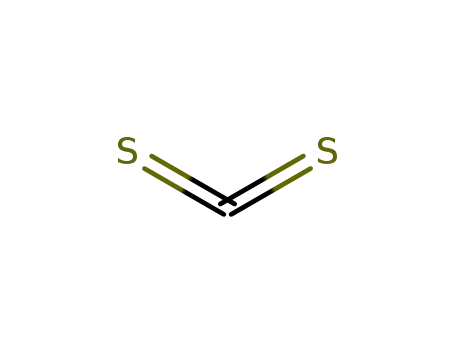
carbon disulfide

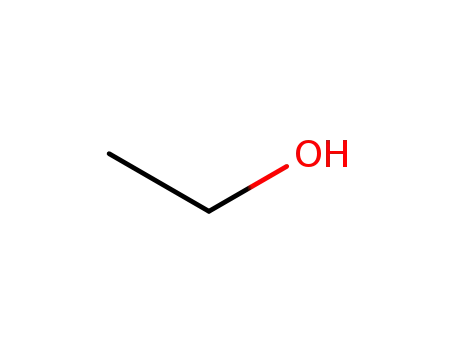
ethanol


bromoacetic acid


2-(ethoxycarbonothioylthio)acetic acid
| Conditions | Yield |
|---|---|
|
carbon disulfide; ethanol;
With
sodium hydroxide;
In
water;
at 0 - 20 ℃;
for 2h;
Inert atmosphere;
bromoacetic acid;
In
water;
at 20 ℃;
for 14h;
Inert atmosphere;
|
75% |

potassium ethyl xanthogenate
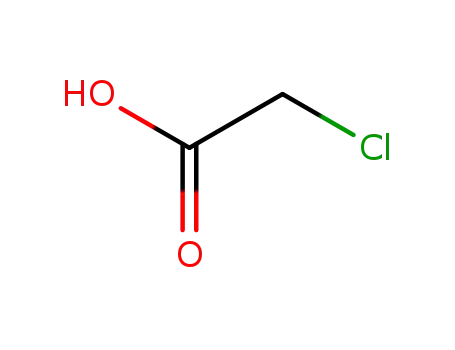
chloroacetic acid
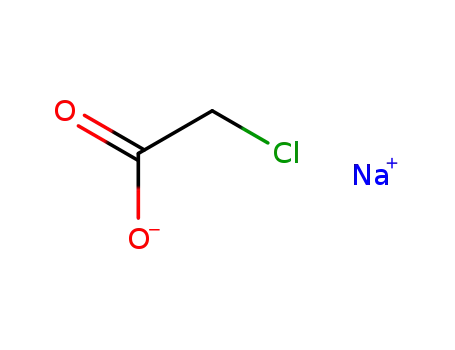
sodium monochloroacetic acid

carbon disulfide
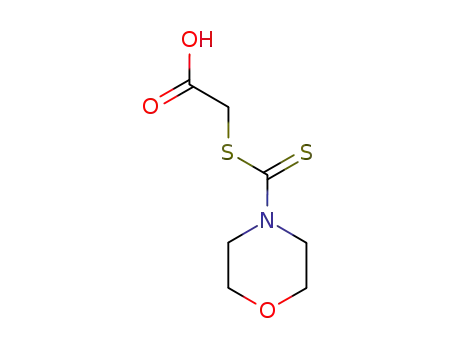
N,N-3-oxapentamethylenethiocarbamoylthioacetic acid
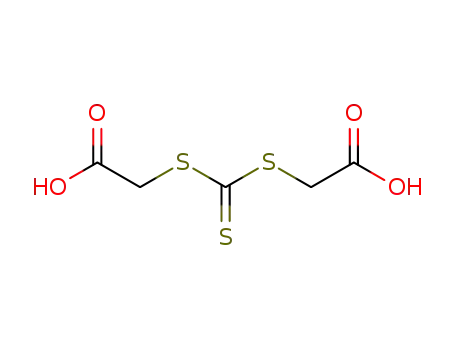
bis(carboxymethyl)trithiocarbonate
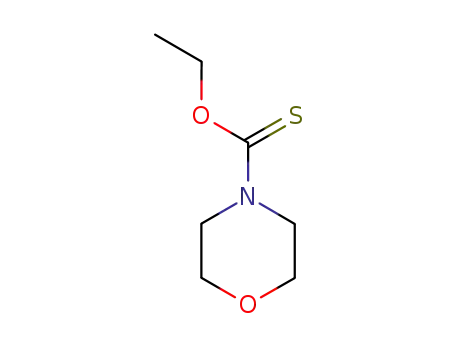
morpholinyl thiocarbamate
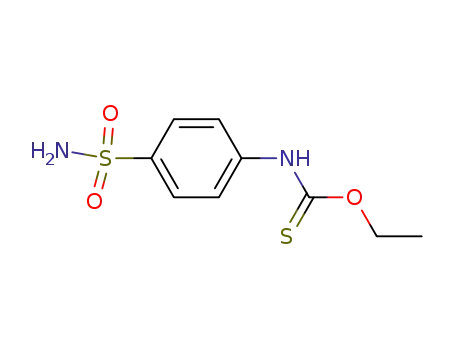
(4-sulfamoyl-phenyl)-thiocarbamic acid O-ethyl ester
CAS:115473-15-9
CAS:1173-88-2
CAS:1204669-58-8
CAS:304896-28-4