- +86-0533-2185556
- WhatsApp: +86 15965530500
- admin@hangyubiotech.com
Your Location:Home >Products >API >62658-63-3

Purity:99%
|
Manufacturing Process |
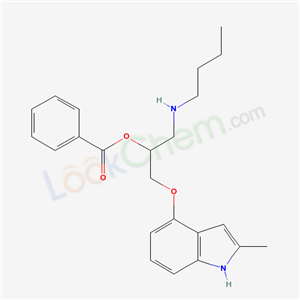
4-(2-Benzoyloxy-3-t-butylaminopropoxy)-2-methyl-indole: 26 g of benzoic acid are dissolved, while heating, in 50 ml of hexamethylphosphoric acid triamide and 3.5 g of 1-t-butylamino-3-(2-methylindole- 4-yloxy)-2-propanol are added. After cooling, 3.0 g of benzoic acid anhydride are added and stirred for 20 hours at room temperature. The resulting clear, yellow solution is poured onto ice 0.5 liters of ether are added and stirred for 2 hours. After making the liquid alkaline with concentrated ammonia, the ether phase is separated, shaken out with tartaric acid, made alkaline with caustic soda solution while cooling with ice and extracted with methylene chloride. After evaporating the solvent, the residue is crystallized with 1 mol of fumaric acid from methanol and acetone. |
|
Therapeutic Function |
Beta-adrenergic blocker |
|
Brand name |
SANDONORM |
InChI:InChI=1/C23H28N2O3/c1-16-13-19-20(25-16)11-8-12-21(19)27-15-18(14-24-23(2,3)4)28-22(26)17-9-6-5-7-10-17/h5-13,18,24-25H,14-15H2,1-4H3
CAS:112163-33-4
CAS:112-84-5
CAS:7300-59-6
CAS:1071-46-1