- +86-0533-2185556
- WhatsApp: +86 15965530500
- admin@hangyubiotech.com
Your Location:Home >Products >Pharmaceutical intermediate >1071-46-1
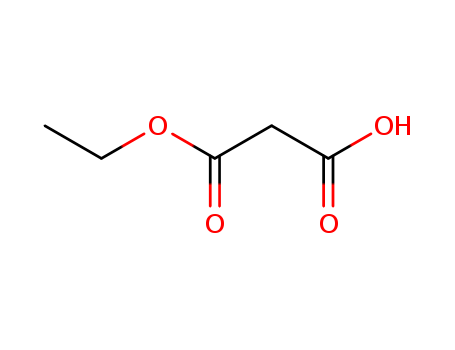

pd_meltingpoint:-13°C(lit.)
Appearance:COA
Purity:99%
|
Synthesis Reference(s) |
Organic Syntheses, Coll. Vol. 4, p. 417, 1963Tetrahedron Letters, 26, p. 1411, 1985 DOI: 10.1016/S0040-4039(00)99058-0 |
InChI:InChI=1/C5H8O4/c1-2-9-5(8)3-4(6)7/h2-3H2,1H3,(H,6,7)
A simple and efficient electrocarboxylat...
The process development of ethyl-(R)-3-a...
2-Alkyloxaziridine-3,3-dicarboxylic acid...
A new stereoselective alkylation methodo...
The highly efficient selective monohydro...
-
Starting from both the bridging nitrogen...
A practical large-scale synthesis of mon...
Boric acid catalyzes the monoesterificat...
Malonic anhydrides decompose at or below...
-
Enantioenriched acyclic α-substituted β-...
A thirteen-step total synthesis of (±)-s...
The present disclosure relates to antiba...
We present an effective deacylative alky...
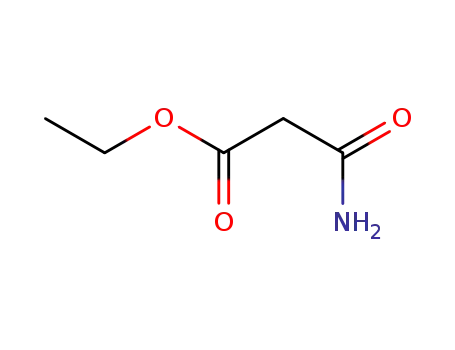
ethyl malonamate

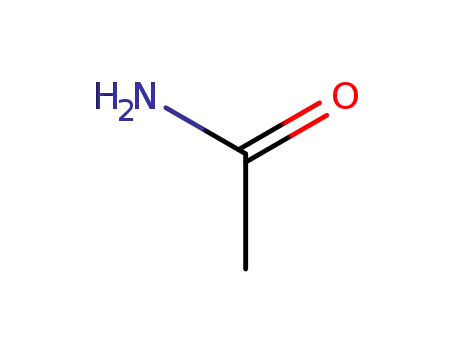
acetamide

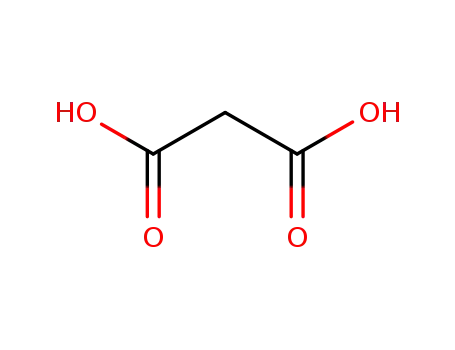
malonic acid

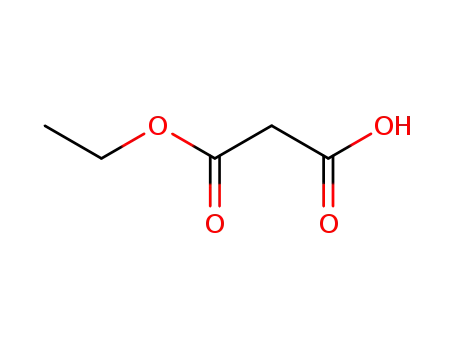
hydrogen ethyl malonate

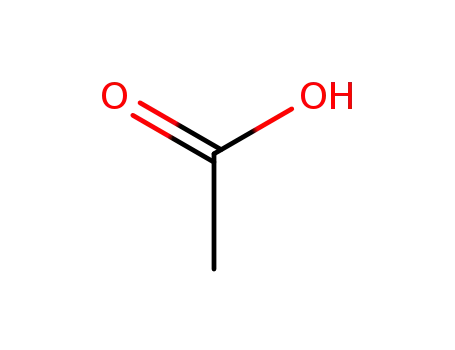
acetic acid
| Conditions | Yield |
|---|---|
|
With
phthalic anhydride;
at 240 - 250 ℃;
for 1h;
under 3040 Torr;
|
53% |
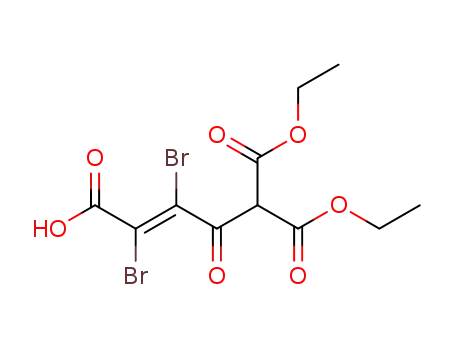
3,4-dibromo-2-oxo-but-3-ene-1,1,4-tricarboxylic acid-1,1-diethyl ester

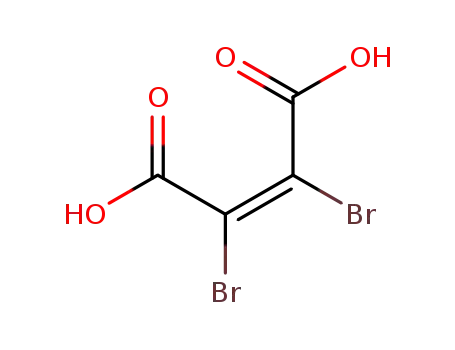
dibromomaleic acid


hydrogen ethyl malonate
| Conditions | Yield |
|---|---|
|
Erwaermen;
|
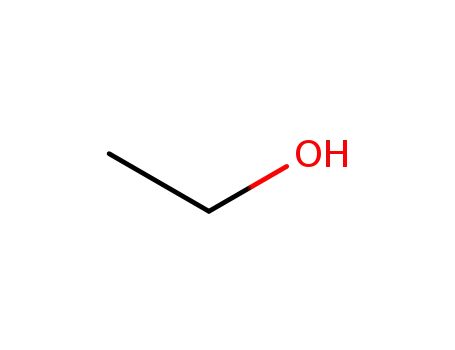
ethanol

malonic acid
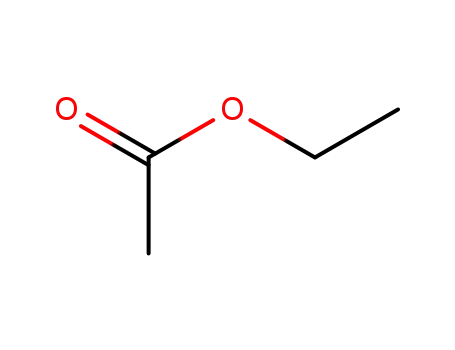
ethyl acetate
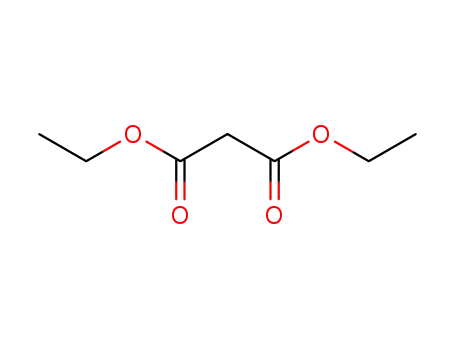
diethyl malonate
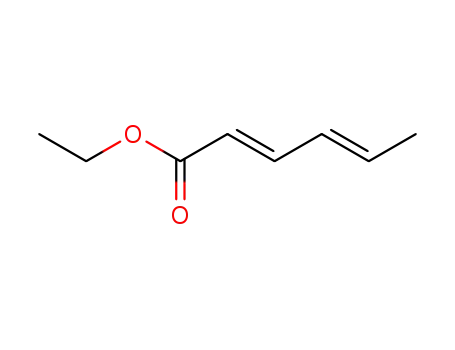
(2E,4E)-ethyl hexa-2,4-dienoate
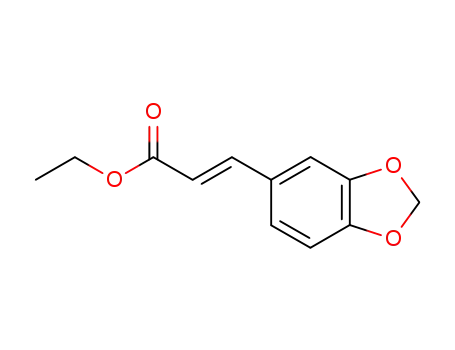
ethyl (E)-3-(benzo[d][1,3]dioxol-5-yl)acrylate
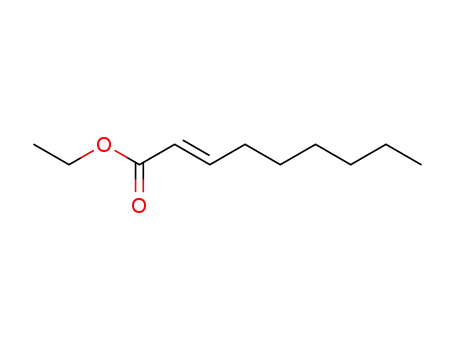
ethyl (2E)-non-2-enoate
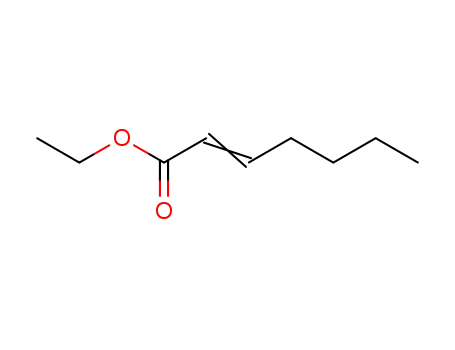
ethyl 2-heptenoate
CAS:112163-33-4
CAS:112-34-5
CAS:62658-63-3
CAS:168749-30-2