- +86-0533-2185556
- WhatsApp: +86 15965530500
- admin@hangyubiotech.com
Your Location:Home >Products >API >220127-57-1
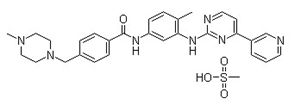

pd_meltingpoint:214-224°C
Appearance:Off-white solid
Purity:99%
|
a small-molecule inhibitor |
Imatinib mesylate (also called Gleevec) is a small-molecule inhibitor of the fusion protein Bcr-Abl, the causal agent in chronic myelogenous leukemia. As an inhibitor of PDGFR, imatinib mesylate appears to have utility in the treatment of a variety of dermatological diseases. Imatinib has been reported to be an effective treatment for FIP1L1-PDGFRalpha+ mast cell disease, hypereosinophilic syndrome, and dermatofibrosarcoma protuberans. |
|
Biochem/physiol Actions |
Imatinib mesylate is a tyrosine kinase inhibitor with antineoplastic activity. Imatinib is a potent inhibitor of the Bcr-Abl kinase encoded by the bcr-abl oncogene as well as receptor tyrosine kinases encoded by c-kit and platelet-derived growth factor receptor (PDGFR) oncogenes. Imatinib mesylate inhibition of Bcr-Abl tyrosine kinase created by the Philadelphia chromosome abnormality found in CML decreases proliferation and enhances apoptosis in leukemias CML and ALL. Inhibition of c-kit tyrosine activity inhibits mast-cell and cellular proliferation in those diseases overexpressing c-kit such as gastrointestinal stromal tumor (GIST). |
|
Molecular Formula and Drug |
Imatinib mesylate (IMAT) is a drug used to treat chronic myeloid leukemia (CML) and gastrointestinal stromal tumors. Its molecular formula is 4-[(4-methylpiperazin-1-yl) methyl]-N- [4-methyl-3-[(4-pyridin-3-ylpyrimidin-2-yl) amino] phenyl]benzamide. IMAT inhibits tyrosine kinase, altering the management of tumors resistant to cytotoxic chemotherapy. |
|
Mechanism of Action |
IMAT functions as a tyrosine kinase inhibitor, specifically targeting BCR-ABL and c-KIT kinases. It inhibits tumor growth and induces apoptosis in cancer cells by impeding the activity of various tyrosine kinases involved in tumor development and blood vessel formation, including PDGFR and VEGFR. |
|
Metabolism |
IMAT is primarily metabolized by the enzyme CYP3A4. Patients may exhibit diverse circulating concentrations of IMAT depending on the dosage, highlighting the importance of monitoring plasma IMAT concentrations for clinical outcomes. |
|
Potential Therapeutic Uses |
In addition to its anticancer properties, IMAT has shown potential anti-Alzheimer's activity, leading to its repurposing for Alzheimer's disease treatment. Liposomal delivery of IMAT via the nose-to-brain route has demonstrated improved drug deposition and residence time in the brain compared to oral and intranasal administration. |
|
Effectiveness and Safety |
IMAT has demonstrated effectiveness as a treatment for colon cancer in preclinical studies and clinical trials. However, its standalone efficacy is limited by the development of drug resistance and adverse reactions such as edema, nausea, and vomiting. |
|
Nanoparticle-Mediated Delivery |
Nanoparticle-based drug delivery systems have been developed to enhance the effectiveness and safety of IMAT. These systems aim to address concerns such as drug resistance and adverse reactions associated with conventional IMAT therapy. |
|
Definition |
ChEBI: A methanesulfonate (mesylate) salt that is the monomesylate salt of imatinib. Used for treatment of chronic myelogenous leukemia and gastrointestinal stromal tumours. |
|
Brand name |
Gleevec, Glivec |
|
General Description |
Imatinib is available in 100- and 400-mg capsules for oraladministration and is indicated for the treatment of CML,gastrointestinal stromal tumors (GIST) that express Kit andacute lymphoplastic leukemia that is positive for thePhiladelphia chromosome.Bioavailability of the agent is nearly 100% by the oralroute. The agent is highly protein bound and metabolized tothe N-desmethyl derivative by CYP3A4-mediated removalof the piperazinyl methyl group. The resulting metabolite issimilar to the parent in activity. Elimination occurs primarilyin the feces, and the terminal half-life is 18 hours forthe parent and 40 hours of the N-desmethyl metabolite.Resistant forms of the TK are known, which have alteredamino acids that prevent binding. In addition, there may beincreased levels of the kinase itself. The drug is also a substratefor Pgp and an additional efflux transporter known asbreast cancer resistance protein (BCRP), both of which removethe drug from the cell. These transporters are also inhibitedby the agent as well. Severe side effects include ascites,neutropenia, thrombocytopenia, skin rash, andpulmonary edema. Less serious side effects include nausea/vomiting, heartburn, and headache but overall, the agentis better tolerated than most other medications used in treatingthe disease. |
InChI:InChI=1/C29H31N7O.CH4O3S/c1-21-5-10-25(18-27(21)34-29-31-13-11-26(33-29)24-4-3-12-30-19-24)32-28(37)23-8-6-22(7-9-23)20-36-16-14-35(2)15-17-36;1-5(2,3)4/h3-13,18-19H,14-17,20H2,1-2H3,(H,32,37)(H,31,33,34);1H3,(H,2,3,4)
The present invention relates to a proce...
The invention relates to a synthesis met...
The invention discloses a preparation me...
The invention relates to the field of dr...
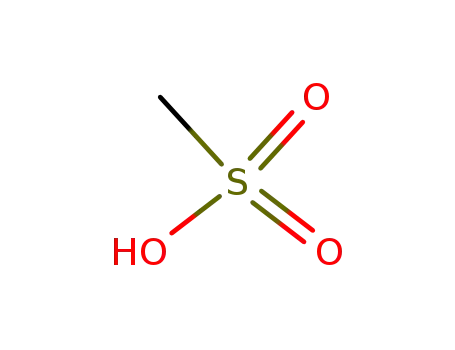
methanesulfonic acid

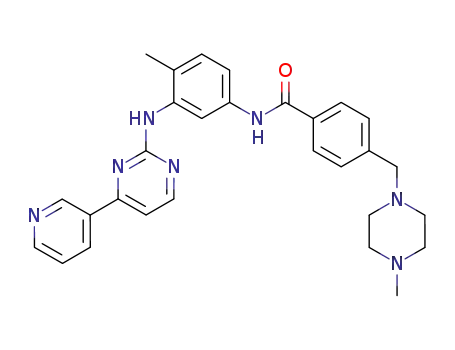
imatinib

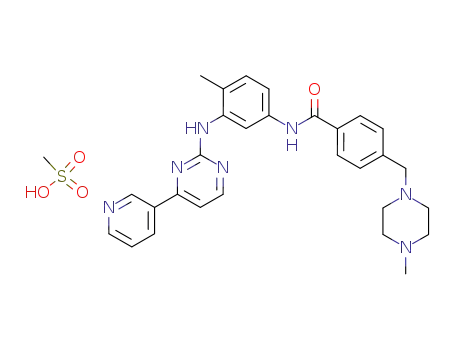
imatinib mesylate
| Conditions | Yield |
|---|---|
|
In acetone; at 55 - 60 ℃; for 2h; Concentration; Temperature; Time;
|
99.89% |
|
In di-isopropyl ether; isopropyl alcohol; at 20 ℃; for 3.16667h; Product distribution / selectivity; Reflux;
|
97% |
|
In isopropyl alcohol; acetone; for 3h; Reflux;
|
97.1% |
|
In isopropyl alcohol; at 65 - 75 ℃; Product distribution / selectivity;
|
96.3% |
|
In water; isopropyl alcohol; at 20 ℃; Product distribution / selectivity;
|
96.6% |
|
In isopropyl alcohol; at 70 ℃; for 0.5h;
|
96% |
|
In 1,2-dimethoxyethane; tert-butyl methyl ether; at 10 - 40 ℃; Product distribution / selectivity;
|
95% |
|
In 1,2-dimethoxyethane; tert-butyl methyl ether; at 10 - 40 ℃; Product distribution / selectivity;
|
95% |
|
In ethyl acetate; at 40 - 60 ℃; Product distribution / selectivity;
|
95% |
|
In chloroform; at 50 ℃; for 5h; Temperature;
|
95% |
|
In acetonitrile; at 25 - 30 ℃; for 0.5h;
|
94% |
|
In 4-methyl-2-pentanone; acetone; at 60 - 65 ℃; for 3.5h; Product distribution / selectivity;
|
94% |
|
In chloroform; water; isopropyl alcohol; at 60 - 70 ℃;
|
94.9% |
|
In ethanol; isopropyl alcohol; at 24 - 70 ℃; for 0.166667h; Product distribution / selectivity;
|
93.6% |
|
In nitromethane; at 90 ℃; Product distribution / selectivity;
|
93% |
|
In isopropyl alcohol; at 74 - 80 ℃; for 1h; Solvent; Temperature;
|
93% |
|
In 4-methyl-2-pentanone; at 65 ℃; for 4h; Product distribution / selectivity;
|
92% |
|
In ethanol; at 60 - 78 ℃; for 1.33h; Autoclave;
|
92.6% |
|
In acetone; at 20 ℃; for 1.5h; Product distribution / selectivity;
|
90% |
|
In acetonitrile; at 15 ℃; for 5h; Product distribution / selectivity;
|
90% |
|
In ethanol; at -40 - -28 ℃; for 14.5h; Product distribution / selectivity;
|
90.1% |
|
In ethanol; water; at -10 - -5 ℃; for 0.333333h;
|
89% |
|
In ethanol; water; at -10 - -5 ℃; for 0.333333h; Product distribution / selectivity;
|
89% |
|
In acetonitrile; at 20 ℃; for 3.5h; Product distribution / selectivity;
|
88% |
|
In dimethyl sulfoxide; at 40 - 45 ℃; Product distribution / selectivity;
|
88% |
|
In isopropyl alcohol; for 3h; Reflux;
|
88% |
|
In acetonitrile; at 20 ℃; for 2.75h; Product distribution / selectivity; Heating / reflux;
|
86.6% |
|
In butanone; at 75 - 77 ℃; for 2h; Product distribution / selectivity;
|
86.5% |
|
In methanol; isopropyl alcohol; at 20 - 85 ℃; for 7.5h; Product distribution / selectivity; Heating / reflux;
|
85% |
|
In acetone; at 20 ℃; for 1.75h; Product distribution / selectivity; Heating / reflux;
|
85% |
|
In acetone; at 20 ℃; for 1.75h; Product distribution / selectivity; Heating / reflux;
|
85% |
|
In ethanol; water; at -27 - -5 ℃; Product distribution / selectivity;
|
85% |
|
In water; isopropyl alcohol; at -28 - -1 ℃; for 0.333333h; Product distribution / selectivity;
|
85% |
|
In ethanol; tert-butyl methyl ether; water; at -27 - -5 ℃; for 3.41667 - 4.75h; Product distribution / selectivity;
|
85% |
|
In methanol; dichloromethane; at 20 ℃; for 3.83333h; Product distribution / selectivity; Heating / reflux;
|
83% |
|
In propan-1-ol; acetic acid; at 82 ℃; under 15.0015 Torr; Product distribution / selectivity;
|
83% |
|
In dimethyl sulfoxide; isopropyl alcohol; at 90 - 95 ℃;
|
80% |
|
In tetrahydrofuran; at 10 - 20 ℃; for 0.666667h; Product distribution / selectivity;
|
76% |
|
In isopropyl alcohol; at 20 - 80 ℃; for 1.91667 - 2.25h; Product distribution / selectivity;
|
72% |
|
In isopropyl alcohol; at 20 - 80 ℃; for 0.333333 - 1.58333h; Product distribution / selectivity;
|
71% |
|
In isopropyl alcohol; at 20 - 80 ℃; for 1.58333h; Product distribution / selectivity;
|
71% |
|
In water; isopropyl alcohol; at 20 ℃; for 0.5h; Product distribution / selectivity;
|
68% |
|
In butan-1-ol; at 70 - 80 ℃; pH=4.5; Inert atmosphere;
|
65.7% |
|
In butan-1-ol; at 70 - 80 ℃; pH=4.5;
|
65.7% |
|
In dioxolane, 1,3-; water; at 5 - 20 ℃; for 0.1667 - 6.25h; Product distribution / selectivity;
|
50% |
|
In ethanol; at 65 - 76 ℃; for 0.0833333 - 0.25h; Product distribution / selectivity;
|
49.1% |
|
In chloroform; at 20 - 50 ℃; for 5 - 41h;
|
|
|
In dichloromethane; at 20 ℃; for 5h;
|
|
|
In tert-butyl alcohol; at 70 - 80 ℃; for 1h; Product distribution / selectivity;
|
|
|
In propan-1-ol; at 70 - 72 ℃; for 0.0833333h; Product distribution / selectivity;
|
|
|
In isopropyl alcohol; for 2h; Heating / reflux;
|
|
|
In 1-methylcyclohexan-4-one; at 65 ℃; for 4h; Product distribution / selectivity;
|
|
|
In ethanol; at 65 ℃; Product distribution / selectivity;
|
|
|
In 1-methylcyclohexan-4-one; at 65 ℃;
|
|
|
In methanol;
|
|
|
In tetrahydrofuran; at 55 - 60 ℃; for 3.5h; Inert atmosphere;
|
|
|
In water; isopropyl alcohol; at -20 - -5 ℃;
|
|
|
In tetrahydrofuran; at 10 - 20 ℃; for 0.666667h; Product distribution / selectivity;
|
|
|
In methanol; at 50 ℃;
|
|
|
In tert-butyl methyl ether; isopropyl alcohol; at 62 - 63 ℃; for 2 - 3h; Product distribution / selectivity;
|
|
|
In methoxybenzene; isopropyl alcohol; at 25 - 85 ℃; for 4.5h; Product distribution / selectivity; Inert atmosphere;
|
|
|
With pyrographite; In methanol; at 50 ℃; for 0.5h; Reflux;
|
|
|
In propan-1-ol; at 0 - 5 ℃; for 0.833333h; Solvent; Temperature; Time; Inert atmosphere;
|
|
|
In ethanol; at 45 ℃; for 2h;
|
|
|
In methanol; dichloromethane; at 25 ℃; for 2h; Inert atmosphere;
|
|
|
In diphenylether; isopropyl alcohol; at 20 - 70 ℃; for 3.5h; Solvent;
|
418.0 g |
|
In water; ethyl acetate; isopropyl alcohol; at 65 - 75 ℃; for 1h; Solvent; Temperature;
|
468 g |
|
In methanol; at 40 ℃; Industrial scale;
|
Ca. 15 kg |
|
In isopropyl alcohol; at 50 ℃; for 1h;
|
4.9 g |

methanesulfonic acid


imatinib

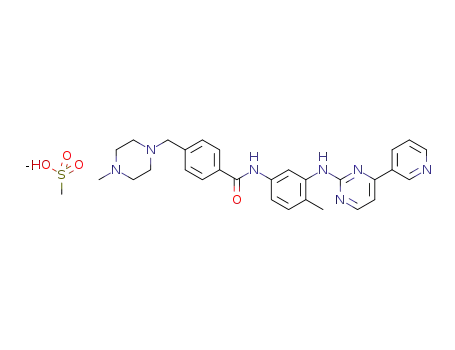
imatinib mesylate
| Conditions | Yield |
|---|---|
|
In isopropyl alcohol; at 20 - 80 ℃; Product distribution / selectivity; Reflux;
|
99.6% |
|
In methanol; at 50 - 55 ℃; for 2.5h; Solvent; Temperature;
|
95% |
|
In methanol; at -10 - 0 ℃; for 0.333333h; Product distribution / selectivity;
|
58% |
|
In ISOPROPYLAMIDE; at 25 - 65 ℃; Product distribution / selectivity;
|
|
|
In N,N-dimethyl acetamide; at 25 - 65 ℃; Product distribution / selectivity;
|
|
|
With potassium mesylate; In water; at 5 - 20 ℃; for 24h; pH=5.2; Product distribution / selectivity;
|
|
|
In water; pH=3 - 3.5;
|

methanesulfonic acid

imatinib
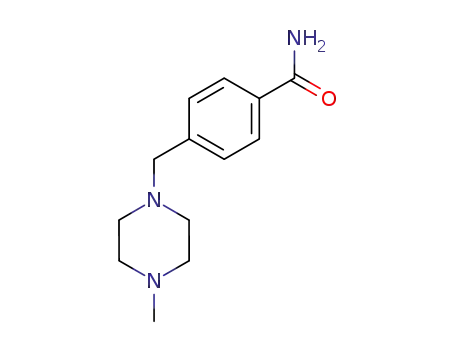
4-((4-methylpiperazin-1-yl)methyl)benzamide
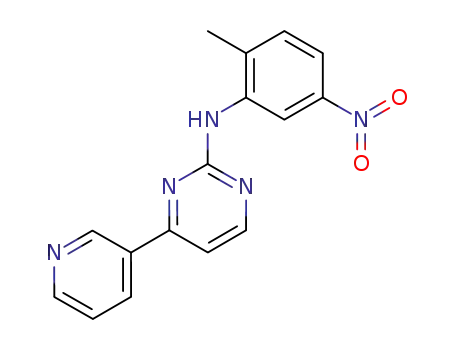
N-(5-nitro-2-methylphenyl)-4-(3-pyridinyl)-2-pyrimidineamine
CAS:118685-33-9
CAS:66104-23-2
CAS:1071-71-2