- +86-0533-2185556
- +86 15965530500
- admin@hangyubiotech.com
Your Location:Home >Products >Pharmaceutical intermediate >1071-71-2
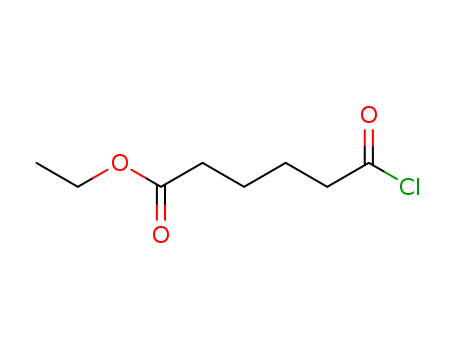

Purity:99%
|
Synthesis Reference(s) |
Journal of the American Chemical Society, 70, p. 3626, 1948 DOI: 10.1021/ja01191a026 |
InChI:InChI=1/C8H13ClO3/c1-2-12-8(11)6-4-3-5-7(9)10/h2-6H2,1H3
TRAP1 (Hsp75) is the mitochondrial paral...
A Gd(III) complex bearing pendant acetox...
Glycoluril derivatives with a carboxylic...
The first enantioselective Ni-catalyzed ...
Triple A: A general asymmetric allylic a...
Prodigiosin is the parent member of the ...
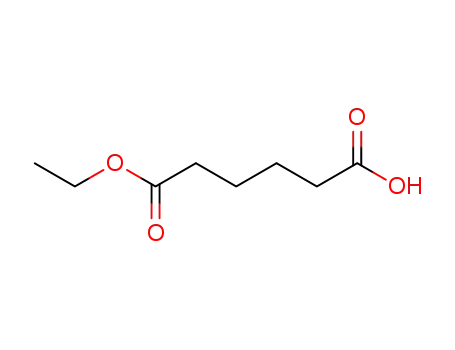
adipinic acid monoethyl ester

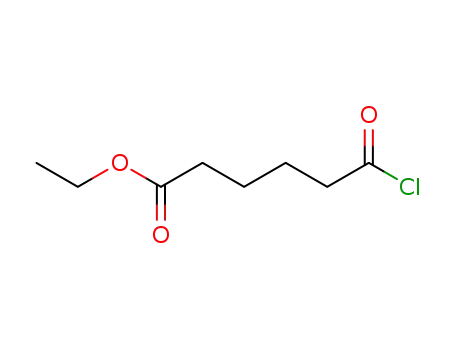
ethyl 6-chloro-6-oxohexanoate
| Conditions | Yield |
|---|---|
|
With
thionyl chloride;
for 1.5h;
Heating;
|
96% |
|
With
oxalyl dichloride;
for 2h;
Heating;
|
73% |
|
With
thionyl chloride;
|
|
|
With
thionyl chloride; benzene;
|
|
|
|
|
|
With
thionyl chloride;
Heating;
|
|
|
With
thionyl chloride;
at 100 ℃;
for 0.5h;
Yield given;
|
|
|
With
thionyl chloride;
for 2.75h;
Heating;
|
|
|
With
thionyl chloride;
|
|
|
With
thionyl chloride;
for 24h;
Heating;
|
|
|
With
thionyl chloride;
at 40 - 50 ℃;
for 3h;
|
|
|
chlorination;
|
|
|
With
oxalyl dichloride;
In
dichloromethane;
Inert atmosphere;
|
|
|
With
oxalyl dichloride; N,N-dimethyl-formamide;
In
dichloromethane;
at 23 ℃;
for 3h;
|
|
|
With
oxalyl dichloride; N,N-dimethyl-formamide;
In
dichloromethane;
at 20 ℃;
|
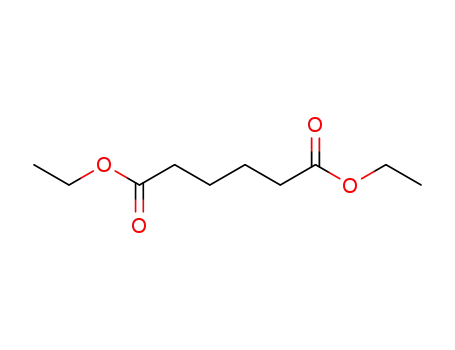
diethyl adipate


ethyl 6-chloro-6-oxohexanoate
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 2 steps
1: 60 percent / KOH / ethanol / 16 h / 0 °C
2: 96 percent / SOCl2 / 1.5 h / Heating
With
potassium hydroxide; thionyl chloride;
In
ethanol;
|

adipinic acid monoethyl ester
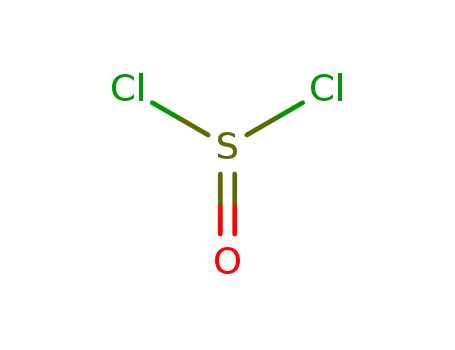
thionyl chloride

diethyl adipate
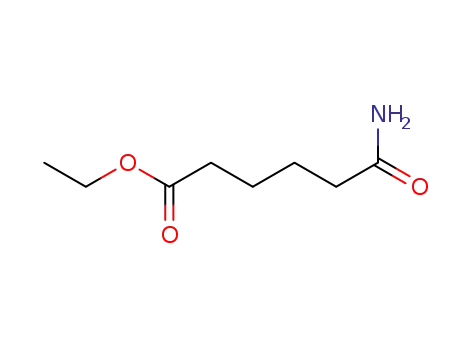
adipic acid ethyl ester-amide
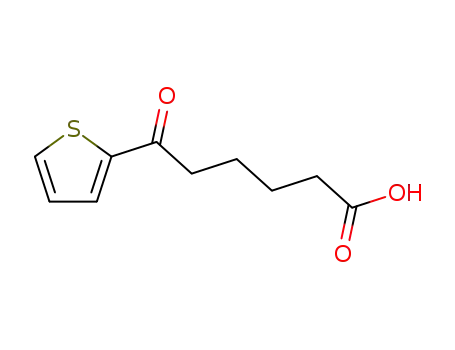
5-Thenoylpentanoic acid
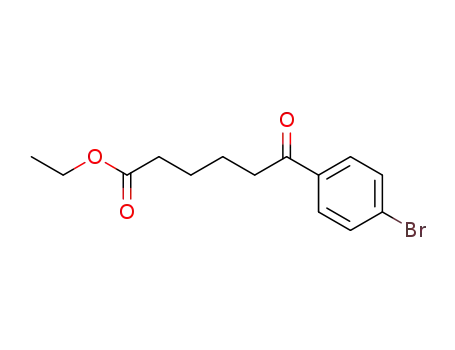
6-(4-bromo-phenyl)-6-oxo-hexanoic acid ethyl ester
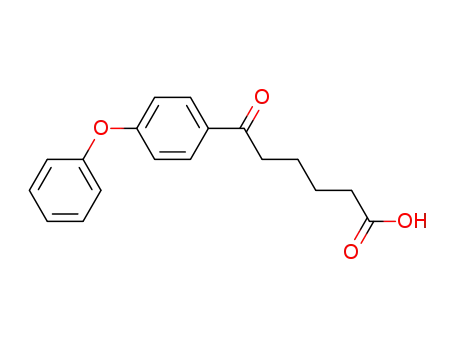
6-oxo-6-(4-phenoxy-phenyl)-hexanoic acid
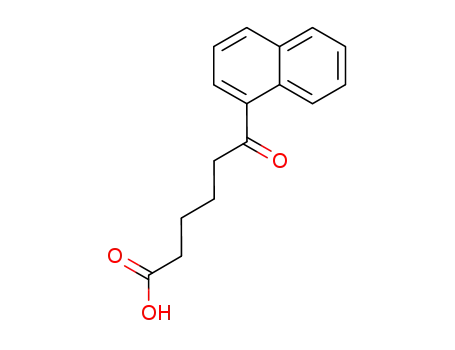
6-[1]naphthyl-6-oxo-hexanoic acid
CAS:115473-15-9
CAS:118685-33-9
CAS:220127-57-1
CAS:865304-71-8