- +86-0533-2185556
- WhatsApp: +86 15965530500
- admin@hangyubiotech.com
Your Location:Home >Products >small-molecule inhibitor >4733-39-5
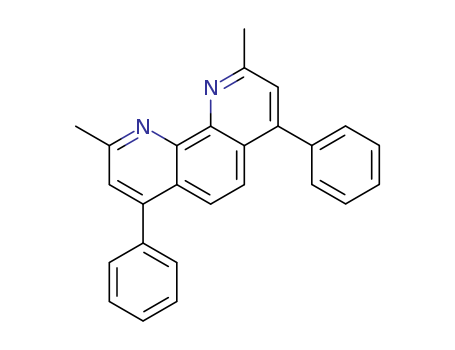

pd_meltingpoint:279-283 ºC
Appearance:yellow powder
Purity:99%
|
Classification |
Electron-transport layer materials, Electron-injection layer materials, Hole-blocking layer materials, OFET, OLED, Organic Photovoltaics, Perovskite solar cells, Sublimed materials. |
|
Applications |
2,9-Dimethyl-4,7-diphenyl-1,10-phenanthroline, also known as Bathocuproine (BCP), is a wide-band-gap material and has a high electron affinity. When it is embedded into organic electronic devices, bathocuproine acts as an exciton-blocking barrier which prohibits exciton diffusion process towards the Al electrode otherwise being quenched. One of the most commonly used buffer layer between acceptor and cathode layers is bathocuproine. The introduction of the buffer layer can greatly improve the PCE of polymer organic solar cells. BCP is one of the most popular hole-blocking layer materials that is used in organic electronics, including perovskite solar cells. It was demonstrated that a BCP buffer layer reduces nonradiative recombination of excitons at the C60 –Al interface. Its most important function is to establish an Ohmic contact between the C60 film and the Al electrode in photovoltaic devices. |
|
General Description |
TGA/DSC Lot specific scans available upon request |
|
Purification Methods |
Purify it by recrystallisation from *benzene. [Smith & Wilkins Anal Chem 25 510 1953, Beilstein 23 III/IV 2160.] |
InChI:InChI=1/C26H20N2/c1-17-15-23(19-9-5-3-6-10-19)21-13-14-22-24(20-11-7-4-8-12-20)16-18(2)28-26(22)25(21)27-17/h3-16H,1-2H3
Ten manganese(I) tricarbonyl diimine com...
The first examples of 4,7-disubstituted ...
C36H25MnN6O3

5-Phenyl-1H-tetrazole

carbon monoxide

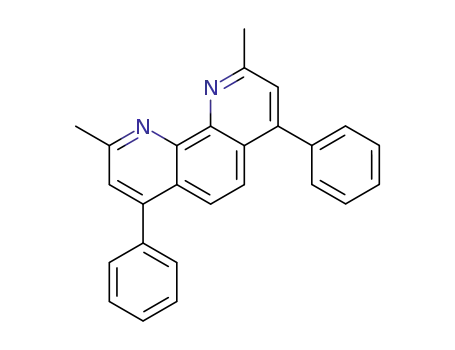
Bathocuproine
| Conditions | Yield |
|---|---|
|
In
dichloromethane;
UV-irradiation;
|
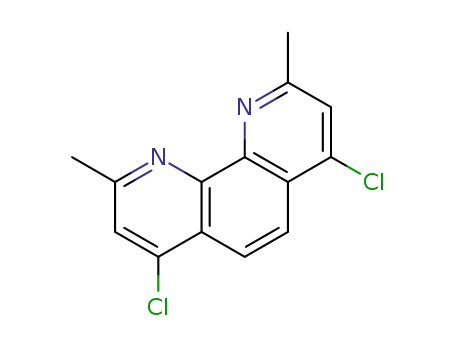
4,7-dichloro-2,9-dimethyl-1,10-phenanthroline

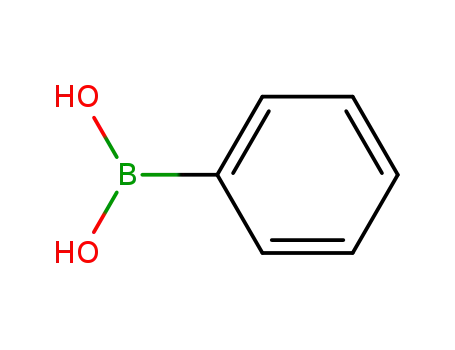
phenylboronic acid


Bathocuproine
| Conditions | Yield |
|---|---|
|
With
tetrakis(triphenylphosphine) palladium(0); potassium carbonate;
In
tetrahydrofuran; water;
for 24h;
Reflux;
Inert atmosphere;
|
98% |
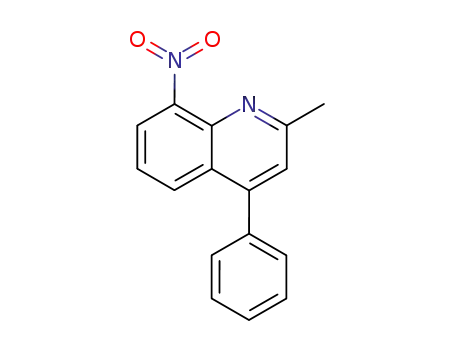
2-methyl-8-nitro-4-phenyl-quinoline
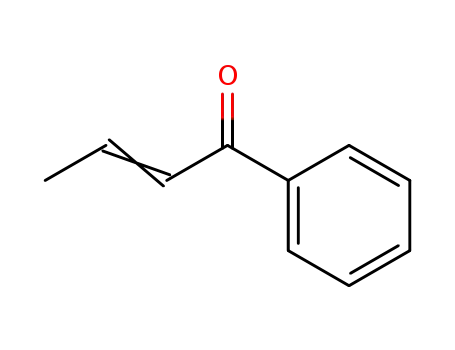
1-phenylbut-2-en-1-one
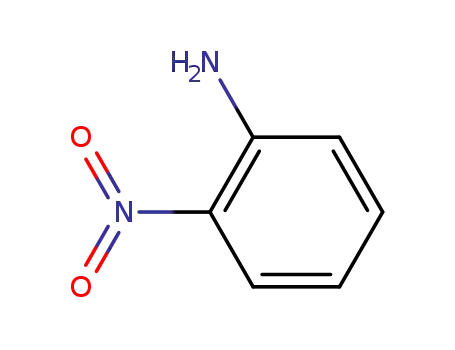
2-nitro-aniline
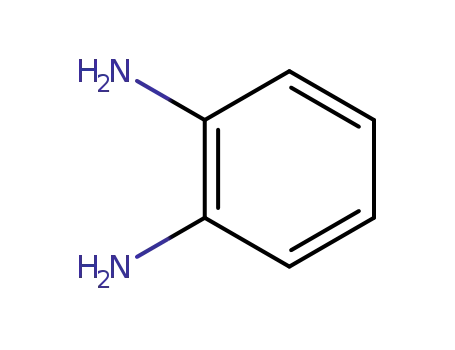
1,2-diamino-benzene
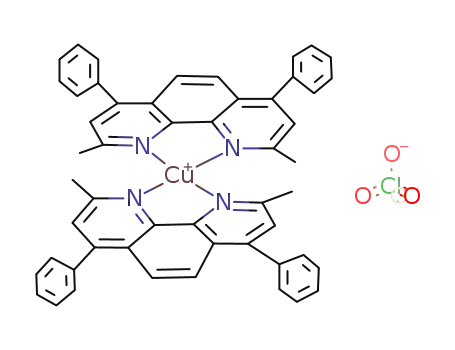
{Cu(bcn)2}ClO4
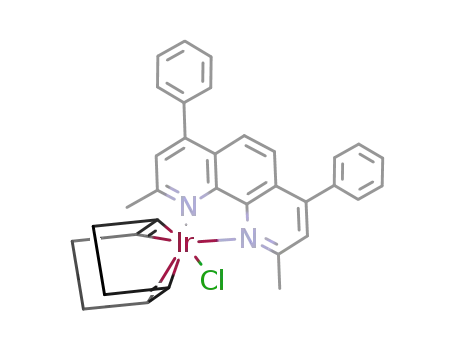
{IrCl(2,9-dimethyl-4,7-diphenyl-1,10-phenanthroline)(1,5-cyclooctadiene)}={Ir(2,9-Me2-4,7-Ph2phen)(COD)}
bis(benzenethiolato)(2,9-dimethyl-4,7-diphenyl-1,10-phenanthroline)zinc(II)
{Re(2,9Me2-4,7-Ph2phen)(CO)3py}*CF3SO3
CAS:112163-33-4
CAS:112-84-5
CAS:107761-42-2
CAS:151271-08-8