- +86-0533-2185556
- WhatsApp: +86 15965530500
- admin@hangyubiotech.com
Your Location:Home >Products >API >151271-08-8

Purity:99%
|
Side effects |
When taken by mouth: Ostarine is possibly unsafe. It might cause liver damage and other serious side effects such as heart attack. |
|
Synthesis |
The synthesis of?imidazenil is as follows:A mixture of 3 g (7.5 mmol) of 6-(2-bromophenyl)-8-fluoro-4H-imidazo[1,5-a][1,4]benzodiazepine-3-carboxylic acid, 300 mL of methylene chloride and 2.25 g (10.8 mmol) of phosphorus pentachloride was stirred at room temperature for 2 hours. Ammonia gas was then introduced until the mixture was basic. After layering with 20 mL of concentrated aqueous ammonia, the mixture was stirred for 15 minutes. The methylene chloride was washed with water, dried and evaporated. The residue was crystallized from ethanol/water to yield 2.4 g of product having the above formula. A second crop of 0.4 g was obtained from the mother liquor for a total yield of 2.8 g. For analysis, a sample of the product was recrystallized from methylene chloride/ethanol and had m.p. 298°-299° C. |
InChI:InChI=1/C18H12BrFN4O/c19-13-4-2-1-3-11(13)16-12-7-10(20)5-6-14(12)24-9-23-17(18(21)25)15(24)8-22-16/h1-7,9H,8H2,(H2,21,25)
The [123I]iodinated analogues of the ben...
The present invention is directed to imi...
![6-(2-bromophenyl)-8-fluoro-4H-imidazo[1,5-a][1,4]benzodiazepine-3-carboxylic acid](/upload/2025/4/07064c5a-7eed-4362-9426-57b25d776f0f.png)
6-(2-bromophenyl)-8-fluoro-4H-imidazo[1,5-a][1,4]benzodiazepine-3-carboxylic acid

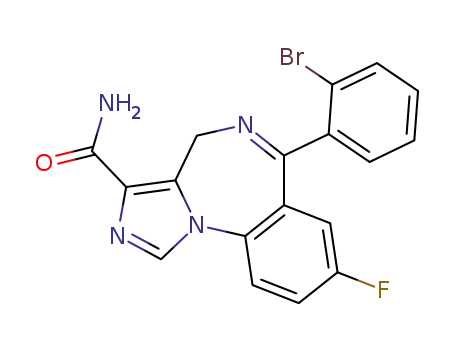
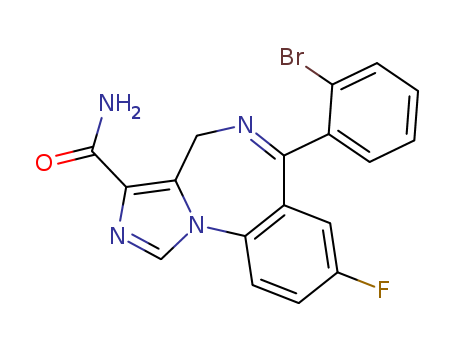
Imidazenil
| Conditions | Yield |
|---|---|
|
With ammonium hydroxide; phosphorus pentachloride; Yield given. Multistep reaction; 1.) CH2Cl2, RT, 2 h, 2.) CH2Cl2, 15 min;
|
|
|
With phosphorus pentachloride; ammonia; In dichloromethane;
|
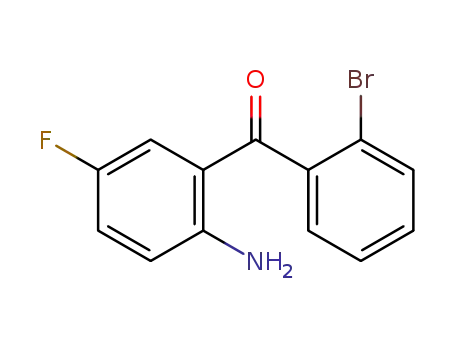
(2-amino-5-fluorophenyl)(2-bromophenyl)methanone


Imidazenil
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 5 steps
1: 88 percent / tetrahydrofuran / 24 h
2: 71 percent / NH2NH2*H2O / methanol / 24 h
3: 1.) potassium tert-butoxide, diethyl chlorophosphate / 1.) THF, from -20 deg C to 10 deg C, 2.) THF, from -20 deg C to RT, 1.5 h
4: 90 percent / 6 N HCl / 24 h / 90 °C
5: 1.) PCl5, 2.) NH3 (gas), NH4OH / 1.) CH2Cl2, RT, 2 h, 2.) CH2Cl2, 15 min
With hydrogenchloride; ammonium hydroxide; phosphorus pentachloride; potassium tert-butylate; diethyl chlorophosphate; hydrazine hydrate; In tetrahydrofuran; methanol;
|
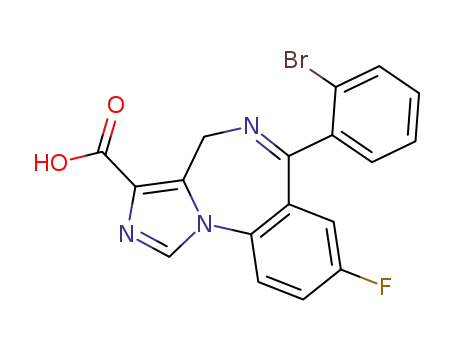
6-(2-bromophenyl)-8-fluoro-4H-imidazo[1,5-a][1,4]benzodiazepine-3-carboxylic acid

(2-amino-5-fluorophenyl)(2-bromophenyl)methanone
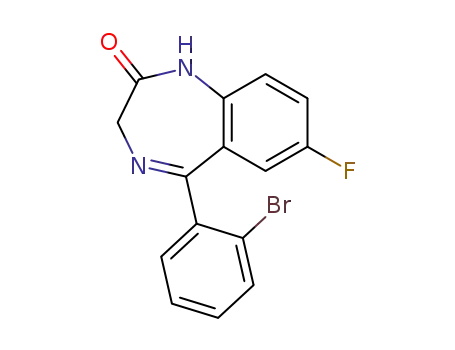
5-(2-bromophenyl)-7-fluoro-1,3-dihydro-1,4-benzodiazepine-2-one
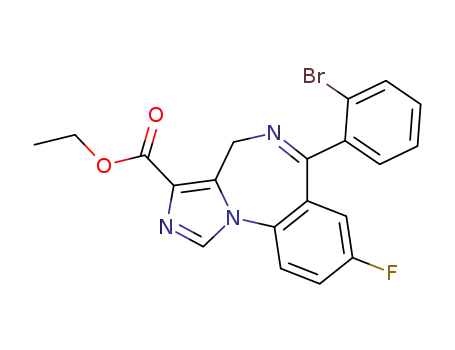
6-(2-bromophenyl)-8-fluoro-4H-imidazo[1,5-a][1,4]benzo-diazepine-3-carboxylic acid ethyl ester
CAS:1204669-58-8
CAS:65896-14-2
CAS:4733-39-5
CAS:68592-15-4