- +86-0533-2185556
- WhatsApp: +86 15965530500
- admin@hangyubiotech.com
Your Location:Home >Products >small-molecule inhibitor >117241-32-4
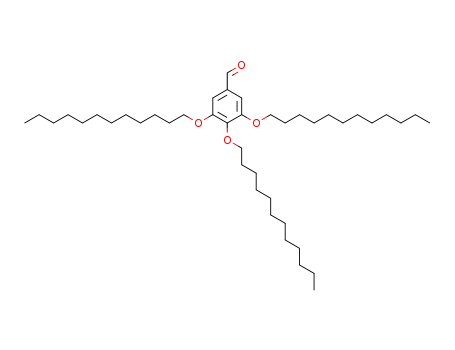

pd_meltingpoint:49.0 to 53.0 °C
Purity:99%
Co-self-assembled vesicular nanoparticle...
We report the synthesis of phenylene(vin...
Photoresponsive materials play an import...
We report the synthesis of trisalkoxy su...
The direct bonding between a thiazolo[5,...
Fluorescent columnar liquid-crystalline ...
Copper and silver nanoparticles were fab...
Tetrathienoanthracene (TTA), a new disco...
Fullerene derivatives bearing long alkyl...
Aggregation induced emission-active bran...
Emissive gold(iii) complexes of pincer 2...
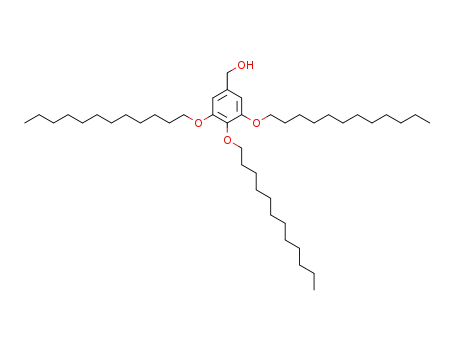
3,4,5-tris(dodecyloxy)benzyl alcohol

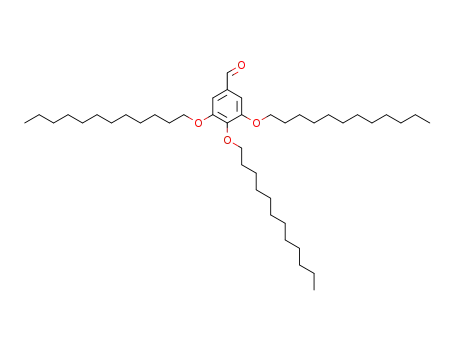
3,4,5-tris(dodecyloxy)benzaldehyde
| Conditions | Yield |
|---|---|
|
With
silica gel; 4-acetylamino-2,2,6,6-tetramethylpiperidine-1-oxoammonium perchlorate;
In
dichloromethane;
|
99% |
|
With
manganese(IV) oxide;
In
dichloromethane;
at 20 ℃;
for 6h;
|
98% |
|
With
pyridinium chlorochromate;
In
dichloromethane;
for 4h;
Inert atmosphere;
Reflux;
|
95% |
|
With
pyridinium chlorochromate;
In
dichloromethane;
at 0 - 25 ℃;
|
90% |
|
With
pyridinium chlorochromate;
In
dichloromethane;
at 0 - 20 ℃;
for 2h;
Inert atmosphere;
|
89% |
|
With
2,3-dicyano-5,6-dichloro-p-benzoquinone;
In
1,4-dioxane;
at 20 ℃;
for 1h;
|
89% |
|
With
manganese(IV) oxide;
In
chloroform;
at 20 ℃;
Inert atmosphere;
|
88.3% |
|
With
pyridinium chlorochromate;
In
dichloromethane;
for 4h;
|
87% |
|
With
pyridinium chlorochromate;
In
dichloromethane;
at 20 ℃;
for 1h;
|
85% |
|
With
pyridinium chlorochromate;
In
dichloromethane;
at 0 - 20 ℃;
Inert atmosphere;
|
85% |
|
With
pyridinium chlorochromate;
In
dichloromethane;
at 20 ℃;
for 3h;
|
84% |
|
With
manganese(IV) oxide;
In
dichloromethane;
at 20 ℃;
for 3h;
|
82% |
|
With
silica gel; pyridinium chlorochromate;
In
dichloromethane;
at 20 ℃;
for 1h;
|
81% |
|
With
pyridinium chlorochromate;
In
dichloromethane;
at 20 ℃;
for 4h;
|
78% |
|
With
pyridinium chlorochromate;
In
dichloromethane;
at 20 ℃;
for 4h;
|
75% |
|
With
pyridinium chlorochromate;
In
dichloromethane;
at 20 ℃;
for 1h;
|
75% |
|
With
pyridinium chlorochromate;
In
dichloromethane;
at 20 ℃;
for 1h;
|
72% |
|
With
pyridinium chlorochromate;
In
dichloromethane;
at 20 ℃;
for 4h;
Inert atmosphere;
|
41% |
|
With
pyridinium chlorochromate;
In
dichloromethane;
for 4h;
Reflux;
|
|
|
With
pyridinium chlorochromate;
In
dichloromethane;
at 0 - 20 ℃;
for 4h;
Inert atmosphere;
|
|
|
With
pyridinium chlorochromate;
In
dichloromethane;
at 0 - 20 ℃;
|
|
|
With
pyridinium chlorochromate;
In
dichloromethane;
at 20 ℃;
for 1h;
|
|
|
With
pyridinium chlorochromate;
In
dichloromethane;
for 3h;
|
|
|
With
pyridinium chlorochromate;
In
dichloromethane;
at 20 ℃;
|
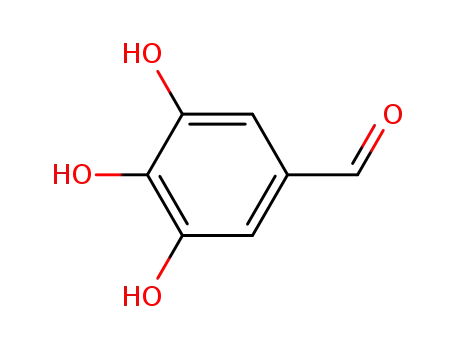
gallaldehyde


1-dodecylbromide


3,4,5-tris(dodecyloxy)benzaldehyde
| Conditions | Yield |
|---|---|
|
With
potassium carbonate; potassium iodide;
In
N,N-dimethyl-formamide;
at 70 ℃;
for 16h;
|
95% |
|
gallaldehyde;
With
potassium carbonate;
In
N,N-dimethyl-formamide;
for 0.25h;
Inert atmosphere;
1-dodecylbromide;
With
potassium iodide;
In
N,N-dimethyl-formamide;
at 80 ℃;
for 12h;
Inert atmosphere;
|
93% |
|
With
potassium carbonate; potassium iodide;
In
N,N-dimethyl-formamide;
at 70 ℃;
for 18h;
|
92% |
|
With
potassium carbonate; potassium iodide;
In
N,N-dimethyl-formamide;
at 70 ℃;
for 14h;
|
91.7% |
|
With
potassium carbonate; potassium iodide;
In
N,N-dimethyl-formamide;
at 160 ℃;
for 15h;
|
90% |
|
With
potassium carbonate;
In
acetonitrile;
|
80% |
|
With
potassium carbonate; potassium iodide;
In
N,N-dimethyl-formamide;
at 80 ℃;
for 24h;
|
78% |
|
With
tetra-(n-butyl)ammonium iodide; potassium carbonate;
In
N,N-dimethyl-formamide;
at 80 ℃;
for 12h;
|
72% |
|
With
potassium carbonate; potassium iodide;
In
acetone;
Heating;
|
35% |
|
With
potassium carbonate; potassium iodide;
In
N,N-dimethyl-formamide;
at 75 ℃;
for 72h;
Inert atmosphere;
|
15% |
|
With
potassium carbonate;
In
butanone;
|
|
|
With
potassium carbonate; potassium iodide;
In
N,N-dimethyl-formamide;
at 70 ℃;
|
|
|
With
potassium carbonate; potassium iodide;
In
butanone;
Reflux;
|
|
|
With
potassium carbonate; potassium iodide;
In
butanone;
|
|
|
With
potassium carbonate;
In
N,N-dimethyl-formamide;
at 90 ℃;
for 18h;
|

gallaldehyde

1-dodecylbromide
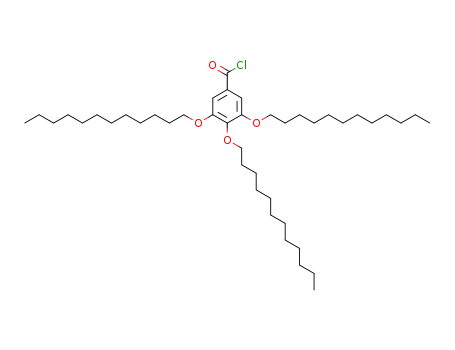
3,4,5-tris(dodecanyloxy)benzoyl chloride

3,4,5-tris(dodecyloxy)benzyl alcohol
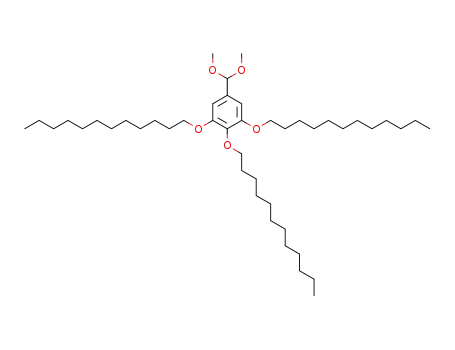
3,4,5-tridodecyloxybenzaldehyde dimethyl acetal
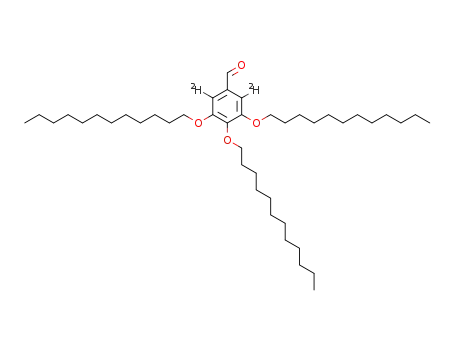
2,6-dideuterio-3,4,5-tridodecyloxybenzaldehyde
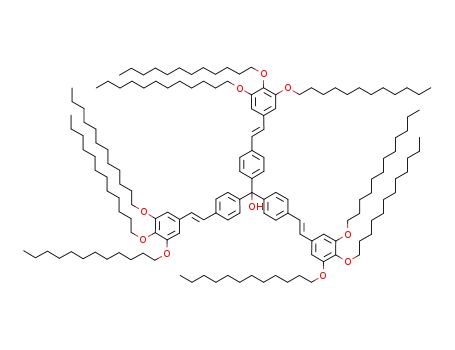
tris(4-{(E)-2-[3,4,5-tri(dodecyloxy)phenyl]ethenyl}phenyl)methanol
CAS:112163-33-4
CAS:112-34-5
CAS:98809-69-9
CAS:1585-90-6