- +86-0533-2185556
- WhatsApp: +86 15965530500
- admin@hangyubiotech.com
Your Location:Home >Products >API >68302-57-8
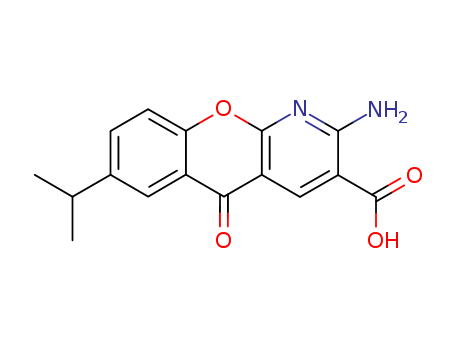

pd_meltingpoint:>300 °C
Appearance:white crystalline solid
Purity:99%
|
Manufacturing Process |
A mixture of 2 ml of morpholine, 3 ml of dimethylformamide and 10 ml of water was heated to 60°C and under stirring the equal molecular quantity of 6-isopropyl-4-oxo-4H-1-benzopyran-3-carbonitrile was added for 5 minutes. The mixture was heated at that temperature for one hour and the resultant precipitate was filtered, rained with water recrystallized from acetic acid and washed with chloroform. By the above procedure was obtained 2-amino-6- isopropyl-4-oxo-4H-1-benzopyran-3-carboxaldehyde melting at 206°-208°C. A mixture of 4 ml ethyl cyanoacetate, 50 ml of ethanol, 5 ml of piperidine and the equal molecular quantity of 2-amino-6-isopropyl-4-oxo-4H-1-benzopyran- 3-carboxaldehyde was refluxed for 30 minutes and, after cooling, the crystalline precipitate was filtered and washed with chloroform. By above procedure was obtained ethyl-2-amino-7-isopropyl-1-azaxanthone-3- carboxylate, melting after recrystallization from ethanol at 243°-244°C. A mixture of 10 ml of acetic acid and 10 ml of 55% sulfuric acid the equal molecular quantity and 2-ethyl-amino-7-isopropyl-1-azaxanthone-3- carboxylate was stirred at 130°C for 4 hours and, after water was added, the precipitate was collected by filtration and recrystalllized from dimethylformamide to give the 2-amino-7-(1-methylethyl)-5-oxo-5H- [1]benzopyrano[2,3-b]pyridine-3-carboxylic acid, melting point 300°C. |
|
Therapeutic Function |
Antiulcer (topical) |
|
Biochem/physiol Actions |
Amlexanox elevates the amount of nonsense-containing mRNAs in treated cells and helps to generate full-length proteins effectively. |
|
Definition |
ChEBI: A pyridochromene-derived monocarboxylic acid having an amino substituent at the 2-position, an oxo substituent at the 5-position and an isopropyl substituent at the 7-position. |
|
Brand name |
Aphthasol (Uluru);Solfa. |
|
General Description |
Amlexanox is an anti-allergic drug with anti-inflammatory properties. |
InChI:InChI=1/C16H14N2O4/c1-7(2)8-3-4-12-9(5-8)13(19)10-6-11(16(20)21)14(17)18-15(10)22-12/h3-7H,1-2H3,(H2,17,18)(H,20,21)
The spherical granules having a core coa...
5-Oxo-5H-[1]benzopyrano[2,3-b]pyridine-3...
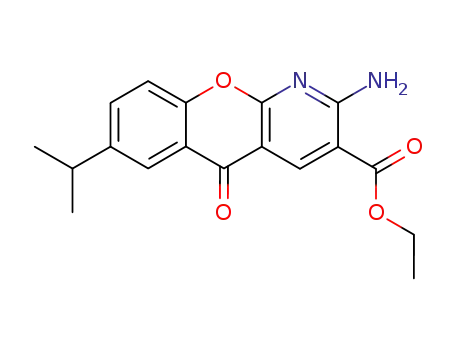
ethyl 2-amino-7-isopropyl-5-oxo-5H-<1>benzopyrano<2,3-b>pyridine-3-carboxylate

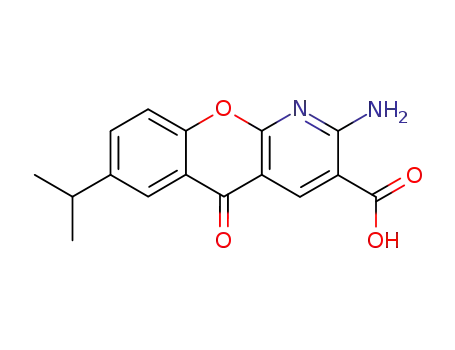
amlexanox
| Conditions | Yield |
|---|---|
|
With sulfuric acid; acetic acid; at 130 ℃; for 3h;
|
88% |
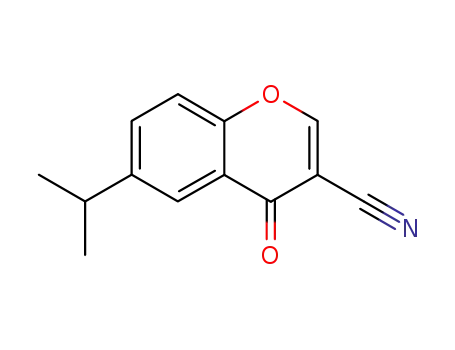
6-isopropyl-4-oxo-4H-1-benzopyran-3-carbonitrile


amlexanox
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 2 steps
1: 95 percent / piperidine / ethanol / 5 h / Heating
2: 88 percent / 50percent aq. H2SO4, AcOH / 3 h / 130 °C
With piperidine; sulfuric acid; acetic acid; In ethanol;
|

ethyl 2-amino-7-isopropyl-5-oxo-5H-<1>benzopyrano<2,3-b>pyridine-3-carboxylate

6-isopropyl-4-oxo-4H-1-benzopyran-3-carbonitrile

ethyl 2-amino-7-isopropyl-5-oxo-5H-<1>benzopyrano<2,3-b>pyridine-3-carboxylate
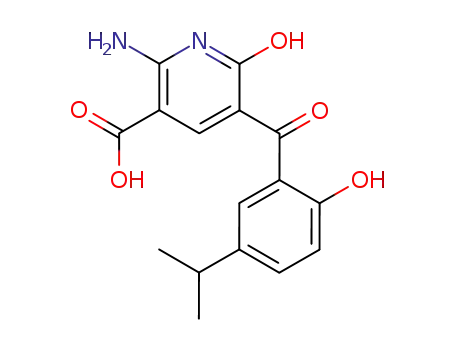
2-amino-6-hydroxy-5-(2-hydroxy-5-isopropylbenzoyl)pyridine-3-carboxylic acid
CAS:112163-33-4
CAS:112-84-5
CAS:10172-75-5
CAS:1445566-01-7