- +86-0533-2185556
- WhatsApp: +86 15965530500
- admin@hangyubiotech.com
Your Location:Home >Products >Pharmaceutical intermediate >10172-75-5
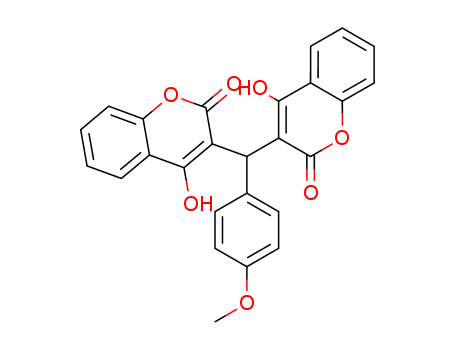

Purity:99%
A series of bisoumarin (1–4) and dihydro...
4-Hydroxycoumarin and Schiff bases conde...
Now a day the derivatives of biscoumarin...
A series of unexpected bis-coumarins hav...
Synthesis of biscoumarin derivatives usi...
The present communication deals with a s...
Chitosan and functionalized graphene oxi...
Abstract: Due to serious side effects of...
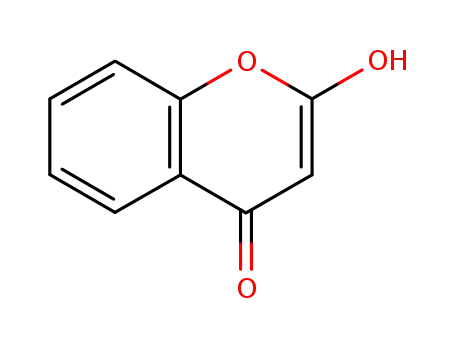
4‐hydroxycoumarin

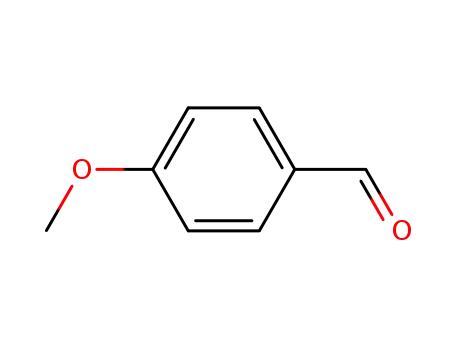
4-methoxy-benzaldehyde

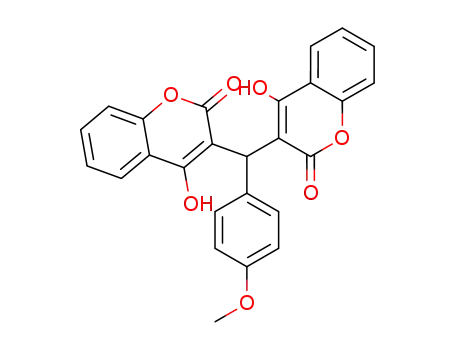
3,3-(4'-methoxybenzylidene)bis(4-hydroxycoumarin)
| Conditions | Yield |
|---|---|
|
With
Ni(II)-dimethylglyoxime complex immobilized on SiO2 coated magnetic Fe3O4 nanoparticles;
In
water;
for 0.05h;
Reflux;
Green chemistry;
|
98% |
|
With
acetic acid;
for 3h;
Reflux;
|
92% |
|
With
Zn-Ni-Fe layered double hydroxide on Fe3O4-graphene oxide nanocomposite;
In
water;
for 0.333333h;
Reflux;
Green chemistry;
|
91% |
|
With
Ni(0) nanoparticles anchored on micro/mesoporous acid-activated montmorillonite;
In
ethanol;
for 0.0833333h;
Microwave irradiation;
|
90% |
|
With
magnetite nanoparticles-supported boron hydrogen sulfate;
In
neat (no solvent);
at 80 ℃;
for 0.166667h;
|
90% |
|
With
ChitosanGOAM-SO3H nanocomposite;
at 80 ℃;
for 0.333333h;
|
88% |
|
With
iron(III) chloride hexahydrate; sodium dodecyl-sulfate;
In
water;
at 100 ℃;
for 0.5h;
Green chemistry;
|
68% |
![4-hydroxy[1]benzopyran-2-one](/upload/2025/4/a7efd362-c34b-4e9c-93a8-ce586de6ccb5.png)
4-hydroxy[1]benzopyran-2-one


4-methoxy-benzaldehyde


3,3-(4'-methoxybenzylidene)bis(4-hydroxycoumarin)
| Conditions | Yield |
|---|---|
|
With
4-sulfophthalic acid;
In
water;
at 80 ℃;
for 0.416667h;
Catalytic behavior;
Green chemistry;
|
100% |
|
With
1,8-diazabicyclo[5.4.0]undec-7-ene;
at 20 ℃;
for 16h;
|
99% |
|
With
phosphotungstic acid;
In
water;
at 80 ℃;
for 0.383333h;
|
99% |
|
With
Sulfonic Acid Functionalized Nanoporous Silica (SBA-Pr-SO3H);
In
ethanol; water;
for 0.5h;
|
99% |
|
With
piperidine;
In
ethanol;
at 20 ℃;
for 4h;
|
98% |
|
With
bismuth(III) vanadate;
In
water;
at 80 ℃;
for 1.5h;
|
98% |
|
With
1,4-diazabicyclo[2.2.2]octane hydroacetate;
In
water;
at 80 ℃;
for 0.05h;
Green chemistry;
|
98% |
|
With
polystyrene functionalized zinc anthranilic acid complex;
In
water;
at 100 ℃;
for 0.05h;
Reagent/catalyst;
Solvent;
Catalytic behavior;
|
98% |
|
With
Zn(II)-grafted benzene-benzylamine-imine-based porous organic polymer;
In
water;
at 80 ℃;
for 0.0333333h;
Catalytic behavior;
Green chemistry;
|
98.5% |
|
In
toluene;
at 90 ℃;
for 0.333333h;
Reagent/catalyst;
|
97% |
|
With
C19H40KNO8S(2+)*2C2F3O2(1-);
In
ethanol; water;
for 0.5h;
Reflux;
|
96% |
|
With
poly(2-acrylamido-2-methylpropanesulphonic acid-co-acrylamide) supported on magnetite nanoparticles;
In
toluene;
at 90 ℃;
for 0.333333h;
|
96% |
|
With
diammonium iron(II) sulfate hexahydrate;
In
water;
for 0.416667h;
Reflux;
Green chemistry;
|
95% |
|
With
cholin hydroxide;
In
water;
at 50 ℃;
for 1h;
|
95% |
|
With
2-(3-phenylthioureido)ethylprolinamide;
In
water;
for 0.25h;
Reflux;
|
95% |
|
With
lemon juice;
In
water;
at 27 - 30 ℃;
for 2h;
|
95% |
|
In
water;
at 95 ℃;
for 5h;
Solvent;
Time;
|
94% |
|
With
Triton X-100;
In
water;
at 20 ℃;
for 7h;
Green chemistry;
|
94% |
|
With
Mn(2,2’-bipyridine-1,1’-dioxide)2Cl2/Mobil Composition of Matter No. 41;
In
water;
for 0.666667h;
Reflux;
Green chemistry;
|
94% |
|
With
bael fruit ash;
In
water;
at 20 ℃;
for 0.25h;
Reagent/catalyst;
Solvent;
Temperature;
Green chemistry;
|
94% |
|
With
1-ethyl-3-(3-sulfopropyl)-benzimidazolium trifluoromethanesulfonate;
at 70 ℃;
for 2.5h;
|
93% |
|
With
30C2H3O2(1-)*72H2O*42H3N*42H(1+)*72Mo(6+)*60Mo(5+)*372O(2-);
In
ethanol;
for 0.133333h;
Reflux;
|
93% |
|
With
C5H9NO*Cl3Zn(1-)*H(1+);
In
neat (no solvent);
at 100 ℃;
for 0.666667h;
Green chemistry;
|
93% |
|
With
nanoparticles of the immobilized Ni(II) species on thiourea functionalized copper ferrite (CuFe2O4SiO2PTMSTuNi(II));
In
neat (no solvent);
at 70 ℃;
for 0.333333h;
|
93% |
|
With
SO3H at Fe3O4 magnetic nanoparticles;
In
neat (no solvent);
at 80 ℃;
for 0.166667h;
|
93% |
|
With
carbon-SO3H;
In
ethanol; water;
at 80 ℃;
for 0.25h;
Green chemistry;
|
93% |
|
With
aluminized polyborate;
In
neat (no solvent);
at 90 ℃;
for 0.583333h;
Green chemistry;
|
93% |
|
With
rhodium(III) chloride hydrate;
In
water;
at 80 ℃;
for 0.5h;
|
92% |
|
With
sulfated titania;
In
water;
at 80 ℃;
for 0.2h;
|
92% |
|
With
sulfonated rice husk ash;
In
water;
at 80 ℃;
for 0.25h;
Green chemistry;
|
92% |
|
With
poly(4-vinylpyridine-co-1-sulfonic acid butyl-4-vinylpyridinium)chloroaluminate;
In
toluene;
at 90 ℃;
for 0.8h;
|
92% |
|
With
Amberlite IRA-400 Cl resin;
In
ethanol; water;
at 80 ℃;
for 2h;
|
92% |
|
With
poly(4-vinylpyridine-co-1-sulfonic acid butyl-4-vinylpyridinium)hydrogen sulfate;
In
toluene;
at 90 ℃;
for 1h;
|
91% |
|
With
C2H8NO7S2(1+)*HO4S(1-);
In
ethanol;
for 0.466667h;
Reflux;
|
91% |
|
With
silica-supported Preyssler acid nanoparticles;
In
ethanol;
at 25 ℃;
for 0.416667h;
|
90% |
|
With
L-tyrosine loaded polymeric nanoparticles;
In
water;
at 70 ℃;
for 0.0833333h;
Reagent/catalyst;
Solvent;
Catalytic behavior;
Green chemistry;
|
90% |
|
With
graphene oxide nanosheets;
In
water;
for 0.3h;
Reflux;
Green chemistry;
|
90% |
|
With
V2O5 based quadruple strontium titanate nanoparticles;
for 0.416667h;
|
90% |
|
With
magnetic nanostructured natural hydroxyapatite;
In
neat (no solvent);
at 90 ℃;
for 1.16667h;
Green chemistry;
|
90% |
|
With
proton exchanged montmorillonite K10;
In
ethanol; water;
at 20 ℃;
for 0.583333h;
|
90% |
|
With
CuFe2O4(at)SiO2(at)3-(2-aminoethylamino)propyl trimethoxysilane(at)Ni(II)nanocomposite;
In
water;
for 0.533333h;
Reagent/catalyst;
Reflux;
Green chemistry;
|
90% |
|
With
4-aminobenzene sulfonic acid;
In
water;
at 80 ℃;
for 0.416667h;
Green chemistry;
|
90% |
|
With
Lipase B Candida Antarctica immobilized on immobead 150 recombinant from yeast;
In
ethanol;
at 20 ℃;
for 9h;
Green chemistry;
|
90% |
|
at 80 ℃;
for 0.5h;
Neat (no solvent);
Ionic liquid;
|
89% |
|
With
poly(4-vinylpyrridine) supported copper(II) oxide nanoparticles;
In
water;
for 0.583333h;
Reflux;
|
89% |
|
With
1H-imidazole;
In
neat (no solvent);
at 60 ℃;
for 0.416667h;
Reagent/catalyst;
|
89% |
|
In
ethanol;
at 20 ℃;
for 2h;
Irradiation;
Green chemistry;
|
89% |
|
With
Thiamine hydrochloride;
In
neat (no solvent);
at 20 ℃;
Green chemistry;
|
89% |
|
With
KF/montmorillonite clay;
In
N,N-dimethyl-formamide;
at 80 ℃;
|
88% |
|
With
bis(tetrabutylammonium) hexatungstate;
In
ethanol;
for 0.0833333h;
Reflux;
|
88% |
|
With
methanesulfonic acid;
In
ethanol;
at 120 ℃;
for 0.133333h;
Microwave irradiation;
|
88% |
|
With
o-benzenedisulfonimide;
In
water;
for 0.416667h;
Reflux;
|
88% |
|
With
caffeine, phosphoric acid;
In
neat (no solvent);
at 80 ℃;
for 1.08333h;
Green chemistry;
|
88% |
|
With
bis(ethylferrocene) modified imidazolium hydrogenoxalate supported on silica coated nanomagnetic Fe3O4;
In
ethanol;
Reflux;
|
88% |
|
With
1-butyl-3-methylimidazolium o-sulfonbenzimide;
In
water;
at 80 ℃;
for 0.416667h;
|
88% |
|
With
1-butyl-3-methylimidazolium Tetrafluoroborate;
at 60 - 70 ℃;
for 3h;
|
87% |
|
With
sodium docusate;
In
water;
at 60 ℃;
for 0.116667h;
Microwave irradiation;
|
87% |
|
With
triethylamine sulfate;
In
ethanol;
at 80 ℃;
for 0.5h;
|
87% |
|
With
acetic acid functionalized poly(4-vinylpyridinum)bromide;
In
neat (no solvent);
at 70 ℃;
for 0.3h;
Green chemistry;
|
87% |
|
With
indium(III) chloride;
In
water;
at 110 ℃;
for 0.333333h;
Microwave irradiation;
Green chemistry;
|
87% |
|
With
sulfosalicylic acid functionalized Fe3O4 nanoparticle;
In
water;
at 60 ℃;
for 0.233333h;
Microwave irradiation;
|
87% |
|
With
ammonium cerium (IV) nitrate;
In
water;
Irradiation;
|
87% |
|
With
silica gel;
for 0.116667h;
microwave irradiation;
|
86% |
|
With
fermented bakers’ yeast;
In
aq. phosphate buffer;
at 20 ℃;
for 0.4h;
Green chemistry;
|
86% |
|
With
N,N,N',N'-tetramethylguanidinium acetate;
at 25 ℃;
for 4.5h;
|
86% |
|
With
lipase from porcine pancreas;
In
ethanol;
at 45 ℃;
for 4h;
Enzymatic reaction;
|
86% |
|
With
ethylenediamine diacetic acid;
In
ethanol;
for 96h;
Ambient temperature;
|
85% |
|
With
propane-1,2,3-triyl tris(hydrogen sulfate);
In
water;
at 80 ℃;
for 0.166667h;
Solvent;
Green chemistry;
|
85% |
|
With
Starch-sulfuric acid;
In
neat (no solvent);
at 80 ℃;
for 0.133333h;
|
85% |
|
With
sulfonic acid-functionalized mesoporous silica nanoparticles;
In
water;
at 80 ℃;
for 0.5h;
Green chemistry;
|
85% |
|
With
silica-bonded 1,4-diaza-bicyclo[2.2.2]octanesulfonic acid chloride nanostructure;
In
neat (no solvent);
at 70 ℃;
for 0.283333h;
|
85% |
|
With
tetrabutylammomium bromide;
In
water;
at 100 ℃;
for 0.5h;
|
84% |
|
With
titanium(IV) oxide;
In
water;
for 0.0833333h;
Reflux;
|
84% |
|
With
copper chromite nanoparticles;
In
water;
at 20 ℃;
for 0.05h;
Green chemistry;
|
84% |
|
With
boron tri(hydrogen sulphate);
In
ethanol; water;
at 70 ℃;
for 0.0833333h;
|
83% |
|
In
water;
at 100 ℃;
Green chemistry;
|
82% |
|
In
ethanol;
for 24h;
Reflux;
|
80% |
|
With
magnetite encapsulated hot-water soluble starch nanoparticles;
In
water;
at 90 ℃;
for 0.716667h;
Green chemistry;
|
80% |
|
With
1,8-diazabicyclo[5.4.0]undec-7-ene functionalized cellulose nanofibers;
In
water;
at 25 ℃;
for 3h;
Green chemistry;
|
80% |
|
In
ethanol;
for 0.05h;
Heating;
|
79% |
|
In
ethanol;
for 5h;
Heating;
|
78% |
|
With
1-n-butyl-3-methylimidazolim bromide;
at 60 ℃;
for 0.666667h;
|
78% |
|
In
water;
at 150 ℃;
for 0.133333h;
Microwave irradiation;
|
76% |
|
With
magnesia;
In
neat (no solvent);
at 100 ℃;
for 0.466667h;
Green chemistry;
|
76% |
|
With
piperidine;
at 90 ℃;
|
66% |
|
With
ethanol;
|
|
|
With
acetic acid;
|
|
|
In
acetic acid;
Heating;
|
|
|
With
hydrogenchloride;
In
ethanol; water;
Reflux;
|
|
|
With
titania sulfonic acid;
In
water;
at 80 ℃;
for 0.666667h;
|
|
|
With
piperidine;
In
ethanol;
at 20 ℃;
for 3h;
|
|
|
With
piperidine;
In
ethanol;
at 20 ℃;
for 3h;
|
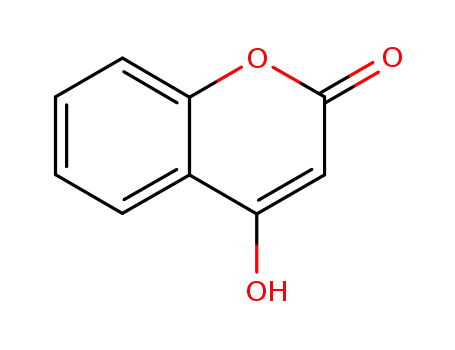
4-hydroxy[1]benzopyran-2-one

4-methoxy-benzaldehyde
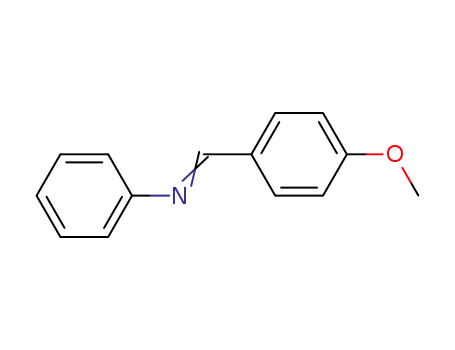
p-methoxybenzylidene-phenylamine
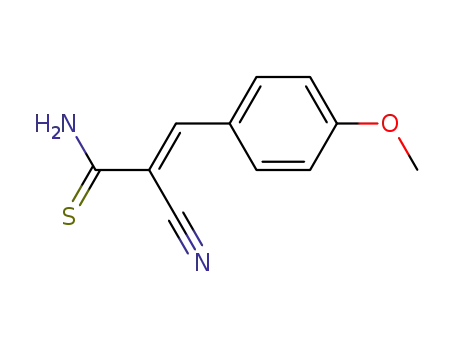
2-cyano-3-(4-methoxyphenyl)thioacrylamide
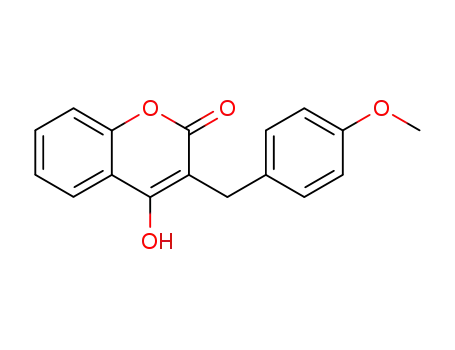
4-hydroxy-3-<(4-methoxyphenyl)methyl>-2H-1-benzopyran-2-one
CAS:112163-33-4
CAS:112-34-5
CAS:872511-34-7
CAS:68302-57-8