- +86-0533-2185556
- WhatsApp: +86 15965530500
- admin@hangyubiotech.com
Your Location:Home >Products >API >53772-83-1
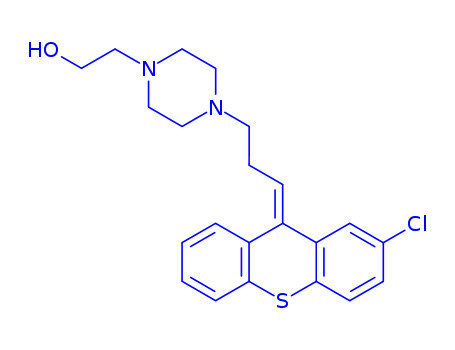

pd_meltingpoint:56-60°C
Purity:99%
|
Drug interactions |
Potentially hazardous interactions with other drugs Anaesthetics: enhanced hypotensive effects. Analgesics: increased risk of convulsions with tramadol; enhanced hypotensive and sedative effects with opioids; increased risk of ventricular arrhythmias with methadone. Anti-arrhythmics: increased risk of ventricular arrhythmias with anti-arrhythmics that prolong the QT interval - avoid with amiodarone and disopyramide. Antibacterials: increased risk of ventricular arrhythmias with moxifloxacin and parenteral erythromycin - avoid Antidepressants: increased level of tricyclics; possible increased risk of convulsions with vortioxetine. Antiepileptics: anticonvulsant effect antagonised. Antimalarials: avoid concomitant use with artemether/lumefantrine. Antipsychotics: avoid concomitant use of clozapine with depot preparations in case of neutropenia; possible increased risk of ventricular arrhythmias with risperidone. Antivirals: concentration possibly increased with ritonavir. Atomoxetine: increased risk of ventricular arrhythmias. Anxiolytics and hypnotics: increased sedative effects. Beta-blockers: increased risk of ventricular arrhythmias with sotalol - avoid. Cytotoxics: increased risk of ventricular arrhythmias with vandetanib - avoid; increased risk of ventricular arrhythmias with arsenic trioxide. |
|
Metabolism |
Metabolism of zuclopenthixol is by sulphoxidation, sidechain N-dealkylation and glucuronic acid conjugation. The sulphoxide metabolites are mainly excreted in the urine while unchanged drug and the dealkylated form tend to be excreted in the faeces. |
|
Definition |
ChEBI: The (Z)-isomer of clopenthixol. |
InChI:InChI=1/C22H25ClN2OS/c23-17-7-8-22-20(16-17)18(19-4-1-2-6-21(19)27-22)5-3-9-24-10-12-25(13-11-24)14-15-26/h1-2,4-8,16,26H,3,9-15H2/b18-5-
Allene (C3H4) gas is produced and separa...

![4-[3-(2-chloro-9H-thioxanthen-9-ylidene)propyl]-1-piperazineethanol](/upload/2025/4/84a71b2a-98eb-4554-9949-dc54bdc85899.png)
4-[3-(2-chloro-9H-thioxanthen-9-ylidene)propyl]-1-piperazineethanol
| Conditions | Yield |
|---|---|
|
2-Chlor-9-<3-piperazino-propyliden>-thioxanthen, Aethylenoxid;
|
|
|
2-Chlor-9-allyl-thioxanthen-9-ol, 1) SOCl2, 2) N-<2-Hydroxy-aethyl>-piperazin;
|
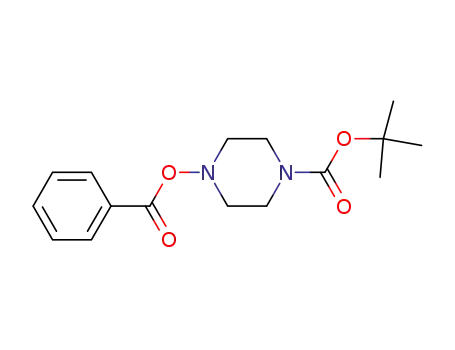
tert-butyl 4-(benzoyloxy)piperazine-1-carboxylate

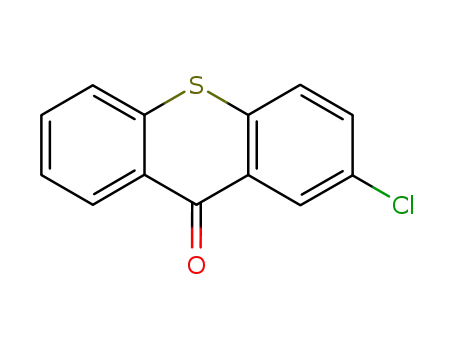
2-Chlorothioxanthone

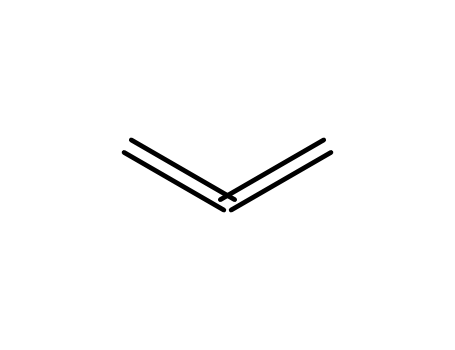
1,2-propanediene

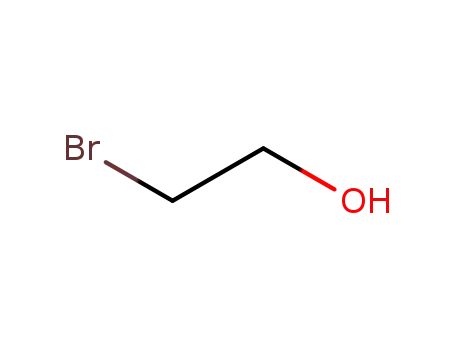
2-bromoethanol

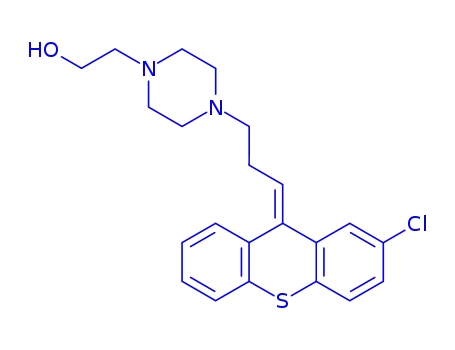
clopenthixol

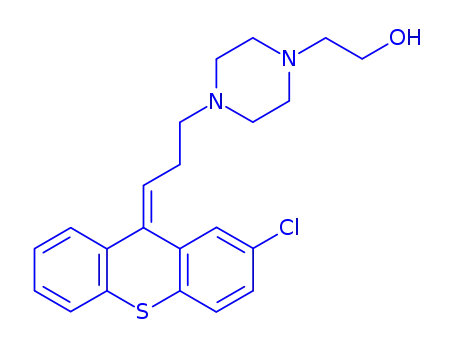
zuclopenthixol
| Conditions | Yield |
|---|---|
|
2-Chlorothioxanthone; With copper diacetate; 2,2'-bis-(diphenylphosphino)-1,1'-binaphthyl; In tetrahydrofuran; for 0.0833333h; Inert atmosphere;
1,2-propanediene; With (dimethoxy)methylsilane; In tetrahydrofuran; at 20 ℃; for 12h; Inert atmosphere; Schlenk technique;
tert-butyl 4-(benzoyloxy)piperazine-1-carboxylate; 2-bromoethanol; Overall yield = 54 %; Overall yield = 217 mg; Further stages;
|
4.762 % de |

tert-butyl 4-(benzoyloxy)piperazine-1-carboxylate

2-Chlorothioxanthone

1,2-propanediene

2-bromoethanol
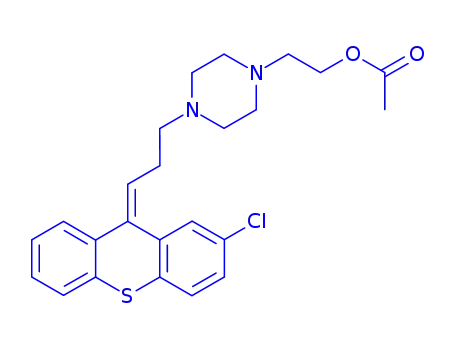
zuclopenthixol acetate
CAS:115473-15-9
CAS:118685-33-9
CAS:763113-22-0
CAS:109-15-9