- +86-0533-2185556
- WhatsApp: +86 15965530500
- admin@hangyubiotech.com
Your Location:Home >Products >API >763113-22-0

Purity:99%
Quality products make an important contribution to long-term revenue and profitability. Factory Supply industrial standard Olaparib 763113-22-0 In Stock
Many of the products generated by alkylating agents on DNA can be efficiently repaired by normal base excision repair (BER). Some poly(ADP-ribose) polymerases (PARPs) assist in the repair of single-strand DNA nicks, an important step in BER. Olaparib is a potent inhibitor of PARP1 and PARP2 (IC50 = 5 and 1 nM, respectively) but is less effective against the PARP tankyrase-1 (IC50 = 1.5 μM). It can be used in cells and in animals, alone or in combination therapy with alkylating agents, to block BER and increase cancer cell death.[Cayman Chemical]
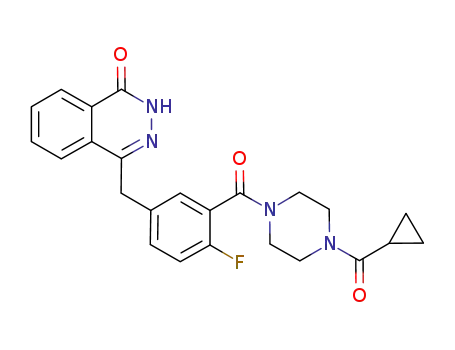
![2-fluoro-5-[(4’-oxo-3’H-phthalazin-1’-yl)methyl]benzoic acid](/upload/2025/4/6f639830-cf3d-48b2-8b8c-545550edbdb1.png)
2-fluoro-5-[(4’-oxo-3’H-phthalazin-1’-yl)methyl]benzoic acid

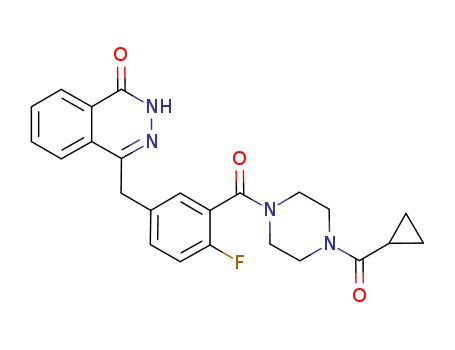
olaparib
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 3 steps
1.1: triethylamine; O-(1H-benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium hexafluorophosphate / N,N-dimethyl-formamide / 1 h / 20 °C / Inert atmosphere
1.2: 72 h / 50 °C / Inert atmosphere
2.1: hydrogenchloride / water; ethanol / 20 °C / Inert atmosphere
3.1: triethylamine; O-(1H-benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium hexafluorophosphate / N,N-dimethyl-formamide / 1 h / 20 °C / Inert atmosphere
3.2: 72 h / 20 °C / Inert atmosphere
With hydrogenchloride; O-(1H-benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium hexafluorophosphate; triethylamine; In ethanol; water; N,N-dimethyl-formamide;
|
|
|
Multi-step reaction with 3 steps
1: thionyl chloride; triethylamine / dichloromethane / 0 - 20 °C
2: isopropyl alcohol / 20 °C
3: acetonitrile
With thionyl chloride; triethylamine; In dichloromethane; isopropyl alcohol; acetonitrile;
|
|
|
Multi-step reaction with 4 steps
1.1: N-ethyl-N,N-diisopropylamine / acetone / 1 h / 20 - 30 °C
2.1: acetone / 1 h / 20 °C
3.1: acetone; water / 4 h / 56.9 - 65 °C
4.1: potassium carbonate / ethyl acetate; water / 1 h / 20 °C
4.2: 2 h / 20 °C
With potassium carbonate; N-ethyl-N,N-diisopropylamine; In water; ethyl acetate; acetone;
|
|
|
Multi-step reaction with 3 steps
1: thionyl chloride; N,N-dimethyl-formamide / 4 h / Reflux
2: dichloromethane / 3 h / -5 - 5 °C
3: triethylamine / dichloromethane / 1 h / -5 - 5 °C
With thionyl chloride; triethylamine; N,N-dimethyl-formamide; In dichloromethane;
|
bromobenzene


olaparib
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 2 steps
1.1: potassium tert-butylate / toluene / 3 h / 90 °C
1.2: 24 h / 110 °C
2.1: C6H11N2(1+)*Cl(1-)*AlCl3 / 50 - 55 °C / Inert atmosphere
With C6H11N2(1+)*Cl(1-)*AlCl3; potassium tert-butylate; In toluene;
|
|
|
Multi-step reaction with 2 steps
1.1: potassium tert-butylate / toluene / 3 h / 90 °C
1.2: 24 h / 110 °C
2.1: C6H11N2(1+)*Cl(1-)*AlCl3 / 50 - 55 °C
With C6H11N2(1+)*Cl(1-)*AlCl3; potassium tert-butylate; In toluene;
|
The CAS number of Olaparib is 763113-22-0.
More information of Olaparib 763113-22-0 are:
|
CAS Number |
763113-22-0 |
|
Density |
1.43 g/cm3 |
|
Refractive Index |
1.702 |
|
HS CODE |
29339900 |
|
PSA |
86.37000 |
|
LogP |
2.22320 |
|
Pka |
12.07±0.40(Predicted) |
Synonyms for Olaparib 763113-22-0:4-[3-(4-Cyclopropanecarbonylpiperazine-1-carbonyl)-4-fluorobenzyl]-2H-phthalazin-1-one;1-(Cyclopropylcarbonyl)-4-[5-[(3,4-dihydro-4-oxo-1-phthalazinyl)methyl]-2-fluorobenzoyl]piperazine;
The chemical formula of Olaparib is C24H23FN4O3 which containing 24 Carbon atoms,23 Hydrogen atoms,1 Fluorine atoms,4 Nitrogen atoms and 3 Oxygen atoms,and the molecular weight of Olaparib is 434.47.
Many of the products generated by alkylating agents on DNA can be efficiently repaired by normal base excision repair (BER). Some poly(ADP-ribose) polymerases (PARPs) assist in the repair of single-strand DNA nicks, an important step in BER. Olaparib is a potent inhibitor of PARP1 and PARP2 (IC50 = 5 and 1 nM, respectively) but is less effective against the PARP tankyrase-1 (IC50 = 1.5 μM). It can be used in cells and in animals, alone or in combination therapy with alkylating agents, to block BER and increase cancer cell death.
InChI:InChI=1/C24H23FN4O3/c25-20-8-5-15(14-21-17-3-1-2-4-18(17)22(30)27-26-21)13-19(20)24(32)29-11-9-28(10-12-29)23(31)16-6-7-16/h1-5,8,13,16H,6-7,9-12,14H2,(H,27,30)
Relevant articles related to Olaparib:
|
Article |
Source |
|
Novel preparation method of olaparib |
- , (2021/08/11) |
|
Preparation method of olaparib |
- , (2019/09/17) |
Zibo Hangyu Biotechnology Development Co., Ltd is a quality supplier of Olaparib. Our main goal is customer satisfaction. Contact us to negotiate the best price for your business on Olaparib 763113-22-0.
CAS:117724-63-7
CAS:391210-10-9
CAS:357336-20-0
CAS:53772-83-1