- +86-0533-2185556
- WhatsApp: +86 15965530500
- admin@hangyubiotech.com
Your Location:Home >Products >small-molecule inhibitor >59981-63-4
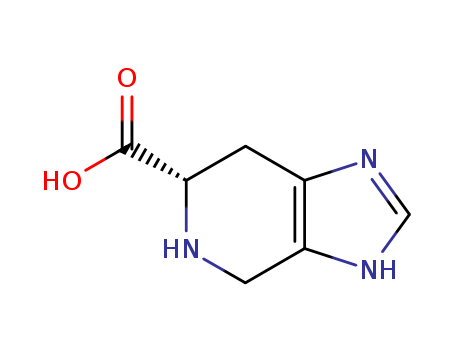

Purity:99%
|
Synthesis Reference(s) |
Journal of Medicinal Chemistry, 27, p. 564, 1984 DOI: 10.1021/jm00371a002 |
InChI:InChI=1/C7H9N3O2/c11-7(12)5-1-4-6(2-8-5)10-3-9-4/h3,5,8H,1-2H2,(H,9,10)(H,11,12)
-
PROBLEM TO BE SOLVED: To provide a novel...
The invention discloses a preparation me...
The invention provides compounds of form...
The invention discloses a hexacyclic pip...
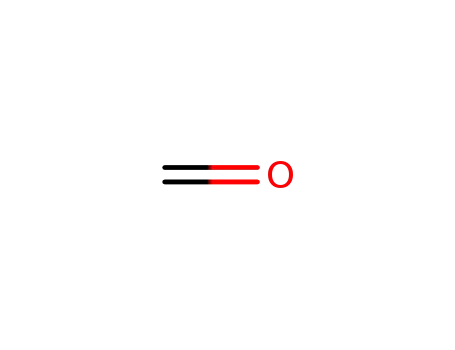
formaldehyd

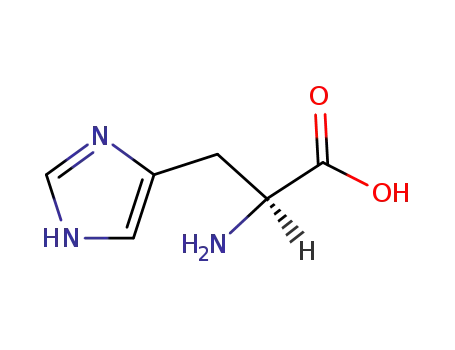
L-histidine

![(6S)-4,5,6,7-tetrahydro-3H-imidazole[4,5-c]pyridine-6-carboxylic acid](/upload/2025/4/4f1f32c2-96a8-41e6-a0e6-79c172982962.png)
(6S)-4,5,6,7-tetrahydro-3H-imidazole[4,5-c]pyridine-6-carboxylic acid
| Conditions | Yield |
|---|---|
|
With
sulfuric acid;
In
water;
at 65 ℃;
for 10h;
Cooling with ice;
|
86% |
|
formaldehyd; L-histidine;
With
sulfuric acid;
In
water;
at 0 - 60 ℃;
for 7h;
With
ammonia;
In
water;
at 0 - 20 ℃;
pH=6;
|
69% |
|
With
sodium dihydrogenphosphate;
|
|
|
With
hydrogenchloride;
In
water;
at 0 - 75 ℃;
for 6.5h;
|
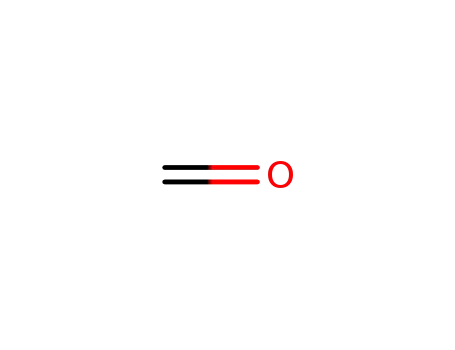
formaldehyd

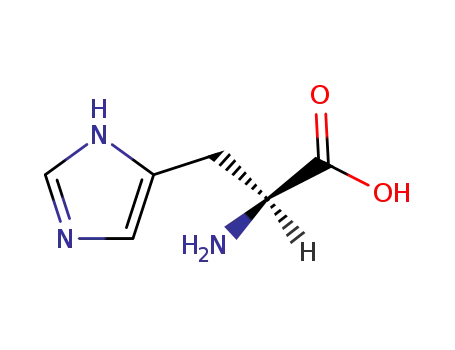
L-histidine

![(6S)-4,5,6,7-tetrahydro-3H-imidazole[4,5-c]pyridine-6-carboxylic acid](/upload/2025/4/4f1f32c2-96a8-41e6-a0e6-79c172982962.png)
(6S)-4,5,6,7-tetrahydro-3H-imidazole[4,5-c]pyridine-6-carboxylic acid
| Conditions | Yield |
|---|---|
|
With
sulfuric acid;
In
water;
at 0 - 60 ℃;
for 6h;
|
98% |
|
With
sulfuric acid;
In
water;
at 65 ℃;
for 10h;
Cooling with ice;
|
86% |
|
With
sulfuric acid;
In
water;
for 5h;
|
70% |
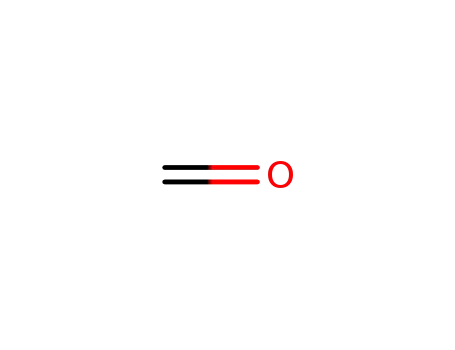
formaldehyd

L-histidine

L-histidine
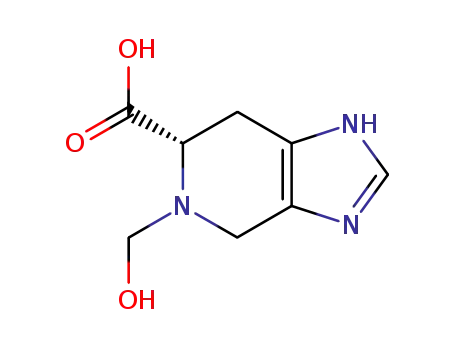
(S)-5-hydroxymethyl-4,5,6,7-tetrahydro-1H-imidazo[4,5-c]pyridine-6-carboxylic acid
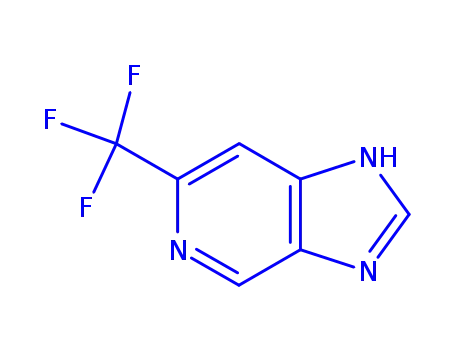
6-(trifluoromethyl)-1H-imidazo<4,5-c>pyridine
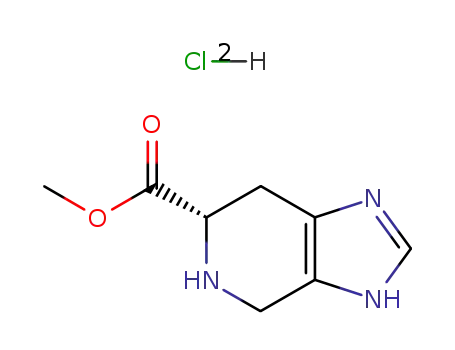
spinacine methyl ester dihydrochloride
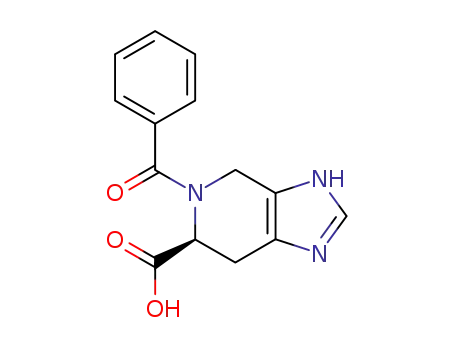
(S)-5-benzoyl-4,5,6,7-tetrahydro-3H-imidazo<4.5-c>pyridine-6-carboxylic acid
CAS:112163-33-4
CAS:112-84-5
CAS:1092351-67-1
CAS:10196-49-3